Research Article Open Access
Chromium(VI) Removal Using Biosorbents Derived from Moringa Oleifera
S Sasikala and G Muthuraman*
Department of Chemistry, Presidency College, Chennai, Tamil Nadu, India
- *Corresponding Author:
- G. Muthuraman
Department of Chemistry
Presidency College, Chennai
Tamil Nadu, India
Tel: +91-044-28544894
E-mail: raman.gm@gmail.com
Received date: September 16, 2015; Accepted date: October 05, 2015; Published date: October 12, 2015
Citation: Sasikala S, Muthuraman G (2015) Chromium(VI) Removal Using Biosorbents Derived from Moringa Oleifera. Ind Chem Open Access 1:105. doi: 10.4172/2469-9764.1000105
Copyright: © 2015 Sasikala S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Industrial Chemistry
Abstract
The increasing influx of heavy metals into water bodies from industrial and domestic activities is of the global concern because of their well-documented negative effects on human and ecosystem health. Moringa oleifera is low cost and environmental friendly material to remove the Cr(VI) from aqueous solution. This study is carried out with the objectives to optimize the feasibility condition of contact time, biosorbents dosage and pH range in removing Cr(VI) by using Moringa oleifera seed powder. The removal characteristics of Cr(VI) by Moringa oleifera was analyzed using UV-Visible spectrophotometer. The optimum percentage removal of Chromium achieved at 120 min at pH 5. The initial metal ion concentration and effect of temperature, Freundlich and Langmuir adsorption isotherm model and kinetics were studied.
Keywords
Environmental; Biosorbents; Moringa oleifera ; Adsorbent dosage
Introduction
Many coagulants are widely used in conventional water treatment processes based on their chemical characteristics. These coagulants are classified into inorganic, synthetic organic polymers and natural coagulants. Coagulation is the most essential process in the treatment of both turbid surface and industrial wastewaters. Coagulationflocculation is one of the most important physicochemical treatment steps in wastewater treatment to reduce the turbidity of wastewater [1,2]. Due to the lack of proper water treatment systems in these rural or under developed communities the best immediate option is to use simple and relatively cost effective point of use technologies such as coagulation [3-5]. Chromium(VI) is highly toxic and soluble in water and it is a strong oxidizing agent that causes severe damage to cell membranes. The most commonly used primary chemical coagulants, namely alum (AlCl3), ferric chloride (FeCl3) and polyaluminium chloride (PAC). The disadvantages associated with these coagulants such as ineffectiveness in low temperature water relatively high procurement cost, detrimental effects on human health, production of large sludge volumes and the fact that they significantly affect pH of treated water. There is strong evidence linking aluminium based coagulants to the development of Alzhemier’s disease in human beings [6]. In addition to these problem chemicals used for water treatment in developing countries constitute a high percentage of annual running expenditure of water treatment companies. The cost of these chemicals in other industrial applications. They are so many methods for removal of Chromium from ground water. The methods are oxidation with chlorine and potassium permanganate treatment with limestone, liquid–liquid extraction, ion exchange, chemical precipitation, bioremediation, use of activated carbon and other filtering materials [7-13].
Moringa oleifera as a coagulant agent provided significant results which justify it is as an alternative coagulant in the process of coagulation/ flocculation of produced water (which is the waste that has the higher volume during the production and exploration of oil [14-16]. Moringa oleifera seed acid extract of natural polyelectrolyte very effective as a coagulant for removal of fluoride from water. Moringa oleifera is the best alternative coagulant that can replace aluminium sulphate [17,18]. In rural areas of the Sudan in particular, the powder seeds of Moringa oleifera are traditionally utilized for water purification because of their strong coagulating properties for sedimentation of suspended mud and turbidity. Many parts of the tree are used as traditional medicines, the seeds contain up to 40% by weight of quality edible oil (greater than 80% unsaturated fatty acid content) and the seeds (and oil free press cake) yield proteins capable of acting as effective coagulants in water and wastewater treatment [19]. The active coagulants of the Moringa oleifera seeds have been determined to be cationic peptides of relatively low molecular weight (6-16 kda) with an isoelectric pH value of 10. In terms of water treatment technologies Moringa oleifera seed in diverse extracted and purified forms has proved to be effective at removing suspended material, generate reduced sludge volumes in comparison to alum soften hard water and act as effective adsorber of cadmium. The use of plant material as natural coagulants to clarify turbidity of wastewater is of common practice [20,21]. Since ancient times powdered roasted grains of Zea mays were used by solider in Peru as a means of settling impurities in the 16th and 17th century. In India ancient writings refer to the use of the seeds of the Nirmali tree strychnos potatorum as a clarifier. The sap of tuna cactus (Opuntia ficus indica) is widely used in chili as water purifying agent. Similarly dried beans (Vicia faba) and peach seeds (Percica vulgaris) are widely used for these purposes in Bolivia and Peru. Moringa oleifera seed act as a natural adsorbents and antimicrobial agent. Its seed Contain 1% active polyelectrolyte’s that neutralize the negative charged colloid in the dirty water. This protein can therefore be a nontoxic natural polypeptide. In the present study the effect of dosage, effect of initial chromium ion concentration, effect of pH, effect of contact time and effect of temperature, Freundlich and Langmuir adsorption isotherm model, Kinetics were studied. Moringa oleifera seed powder is environmental friendly method. Moringa oleifera seed powder used in water treatment it produce large amount of sludge it is used as biofertilizer or biocompost.
Materials and Methods
Materials
All chemicals used were of analytical grade and supplied by Merck (purity >99%). The stock solution of Cr(VI) was prepared at a concentration of 1000 mg/L from potassium dichromate using double distilled water. The pH adjustments were made using 0.1 M NaOH and 0.1 M HCl.
Preparation of Moringa oleifera seed powder: Good quality dry seeds of Moringa oleifera pods that were collected from Tindivanam. The dried Moringa oleifera seeds coat and wings were removed manually followed by the grinding of the seeds into a fine powder using a domestic blender then sieving the griend powder through 250 μm sieve [1].
Equilibrium experiments: Equilibrium studies at different metal ions concentrations were investigated using the batch adsorption process as described earlier. Batch adsorption studies were conducted in a set of 250 ml glass stopper Erlenmeyer flasks containing appropriate dose (0.5 g) of adsorbent and 50 ml of metal ion solution. This mixture solution was agitated at a speed of 500 rpm in a magnetic shaker until the equilibrium was attained. After equilibrium, supernatant was filtered and the equilibrium concentration of Cr(VI) was analyzed, respectively, using UV–Visible spectrophotometer. The percentage metal ion removal (R) was calculated using the following equations:
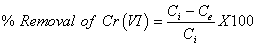 (1)
(1)
Where C0 and Ce are the initial and equilibrium concentrations of the metal ion solution (mg/L), V is the volume of the solution (L), and M is the mass of the adsorbent used (g). The obtained data were fitted into adsorption isotherms, Lagergren’s first-order, Pseudo-secondorder Models.
Results and Discussion
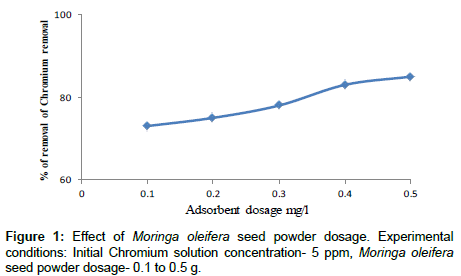
Effect of adsorbent dosage
The relation between the percentage removals of chromium with adsorbent dosage is shown in Figure 1. From this curve it is clear that the percentage removal of chromium increased with the increase of adsorbent dosage the similar result were found that other researchers. The initial chromium concentrations were set at 5 ppm and pH of 5. The Moringa oleifera seed powder was varied between 0.1 g/l to 0.5 g/l. For coagulant dosage of 0.5 g/l achieved percentage reduction of chromium was more than 85%. This result can be explained as when the adsorption dose reached a certain rate the adsorption site was used up, hence with reduced tendency of the particles to adsorb any more ions to its surface. So removal rate of heavy metal ions no longer increased. The similar results were found by other researchers [22-24].
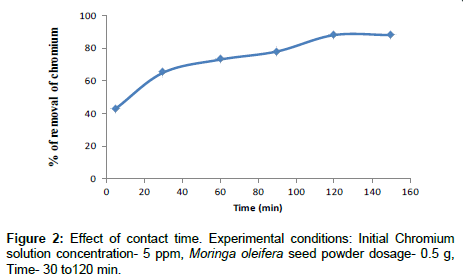
Effect of contact time
The optimum contact time was determined based on the percentage efficiencies. The removal of Cr(VI) from solution as a function of time is presented in Figure 2. It can be seen that the adsorbed amount of metal ions increased by increasing contact time and reached equilibrium at 120 min. Further experiments were carried out as 120 min for contact time. This result may be due to the use of vacant adsorption sites on the adsorbent surface. Large number of vacant adsorbent sites were available for initial stage of sorption. After a lapse in time the remaining vacant surface sites were occupied due to repulsive forces between the solute molecules on the adsorbent surface and the bulk phase. The similar results were obtained by other researcher [25]. At this point the amount of metal being adsorbed onto the biosorbents were in a state of dynamic equilibrium with the amount of adsorb from the biosorbents.
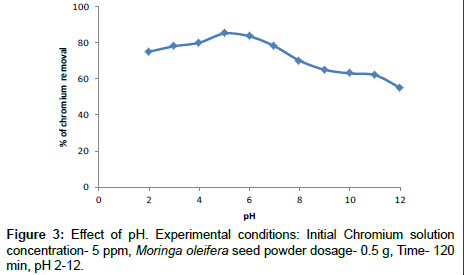
Effect of pH
The effect of pH on the removal of chromium is shown in the Figure 3. It is evident that for Moringa oleifera seed powder the percentage removal of chromium is almost 85% at pH 5. When the basic pH reached 10-13 where a high degree of the precipitation of metal ion can be expected in the insoluble forms. The percentage removal of chromium ions increased at acidic pH values are present H+ and Cr3+ ions compete for the active sites of adsorption. The increasing in pH>8 which has been estimated to occur metal hydroxide. Further experiments were carried out at pH 5. The similar results have been reported by other researchers. So pH 5 was considered as optimum condition and was used for further study [26].
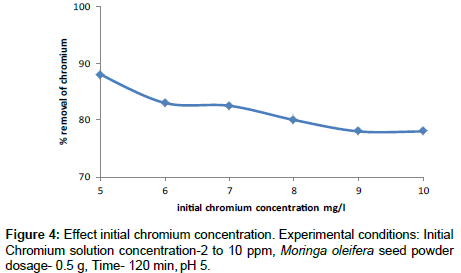
Effect of initial metal ion concentration
The effect of Cr(VI) concentration from 5 to 10 mg/l on the adsorption process has been investigated using 0.5 g of Moringa oleifera seed powder. The percentage removal is achieved at 88% for 5 mg/l at contact time of 120 min. The percentage removal was found to decrease exponentially at high chromium concentrations lack of available active sites is required. Similar results have been reported in previous studies [26]. Moringa oleifera seed powder adsorption and interparticle bridging takes place to remove Cr(VI) from synthetic aqueous solutions (Figure 4).
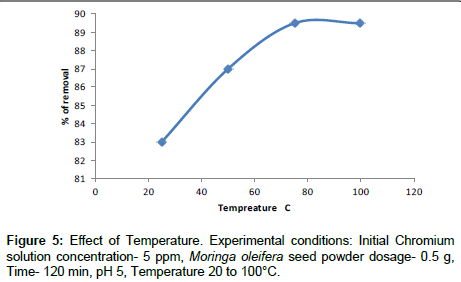
Effect of temperature
The effect of temperature on Cr(VI) adsorption was studied. The experiment were conducted with Cr(VI) concentration of 5 ppm and a Moringa oleifera dosage of 0.250 g at pH 5 for 2 hrs. The temperature varies from 25°C to 100°C, indicating that the adsorption of Cr(VI) onto Moringa oleifera is an endothermic process. The adsorption capacity of Cr(VI) was increased by increasing the temperature (Figure 5).
Adsorption isotherms
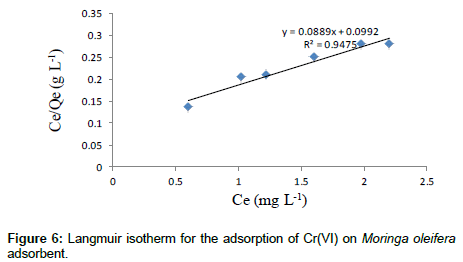
The adsorption studies were conducted at fixed initial concentration of heavymetals by varying adsorbent dosage. The equilibrium data obtained were analysed in the light of Langmuir and Freundlich isotherms. Adsorption isotherm was assayed by studying the adsorption of Cr(VI) with wide concentration range in aqueous solutions onto Moringa oleifera seed adsorbent. The amount adsorbed of metal ion onto carbon adsorbent (mg/g) was represented against the equilibrium concentration of metal ion in aqueous solution. Langmuir isotherm model is based on the assumption that maximum adsorption corresponds to a saturated monolayer of adsorb ate molecules on the adsorbent surface that the energy of adsorption is constant and that there is no transmigration of adsorb ate in the plane of the surface. Adsorption isotherm was obtained by shaking the adsorbent of fixed doses and the adsorbate solution containing varied concentrations of metal ion for 2 h. The Langmuir isotherm model represents the equilibrium distribution of metal ions between the solid and liquid phases. The following equation can be used to describe adsorption isotherm according to Langmuir:
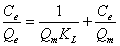 (2)
(2)
Where Ce is the equilibrium concentration (mg L-1), Qe is the amount adsorbed at equilibrium (mg g-1), Qm (mg g-1) and KL (L mg-1) are Langmuir constants related to adsorption capacity and energy of adsorption respectively.
shows the linear plot of Ce/Qe vs. Ce for Moringa oleifera powder. The values of Qm and KL were determined from slop and intercept of the linear plot of Ce/Qe vs. Ce. The experimental data and the correlation coefficients (R2) values of Moringa oleifera seed powder was 0.947 indicates the applicability of the Langmuir isotherm model. The essential feature of Langmuir isotherm can be expressed by means of dimensionless constant referred to as the separation factor or equilibrium parameter, RL which is defined by the following equation [24]:
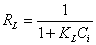 (3)
(3)
Where Ci is the initial metal ion concentration (mg L-1). The value of separation factor RL, indicates the nature of the adsorption process as given below:
| RL value | Nature of adsorption process |
| RL>1 | Unfavorable |
| RL=1 | Linear |
| 0<RL<1 | Favorable |
| RL=0 | Irreversible |
The values of RL values calculated for this study are given in Table 2. The adsorption process will be favorable if the RL values lie between 0 and 1. The RL values given in Table 2, very well lie in this range and hence the adsorption process was favorable [25].
| Cr(VI) concentration (mg L-1) | The RL value |
|---|---|
| 5 | 0.2272 |
| 6 | 0.1893 |
| 7 | 0.1623 |
| 8 | 0.1420 |
| 9 | 0.1262 |
| 10 | 0.1136 |
Table 2: The values of RL values.
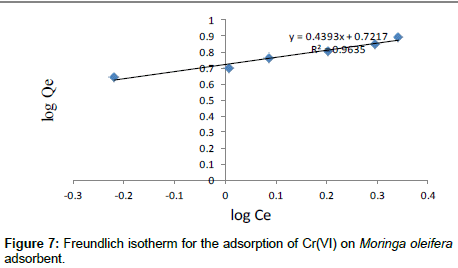
The Freundlich model was chosen to estimate the adsorption intensity of the metal ions on the carbon adsorbent surface. The Freundlich equation is presented as:
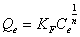 (4)
(4)
This expression can be linearized to give the following equation:
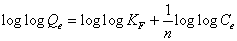 (5)
(5)
Where Kf (mg/g) and n are Freundlich constants incorporating all factors affecting the adsorption process such as adsorption capacity and intensity of adsorption. These constants are determined from the intercept and slope of linear plot of log qe versus log Ce, (Figure 7). Although the correlation coefficients are greater than 95%, they do not correlate the data as well as the Langmuir isotherm, which has consistently higher correlation coefficients.
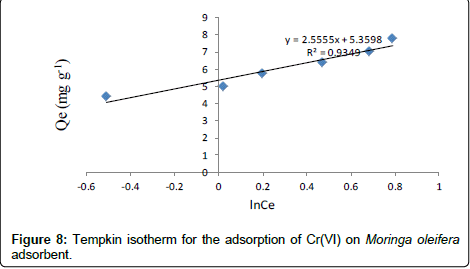
Tempkin isotherm model
Tempkin isotherm contains a factor that explicitly takes into account adsorbing species-adsorbate interactions. This isotherm assumes that the heat of adsorption of all molecules in the layer decreases linearly with coverage due to adsorbate-adsorbate interaction and adsorption is characterized by a uniform distribution of binding energies, up to some maximum binding energy. Tempkin isotherm has generally been used in the linearized and rearranged form as following: [26,27]
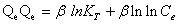 (6)
(6)
Where, KT is an equilibrium constant of binding corresponding to the maximum energy of binding (mg L-1) and the β is related to the heat of adsorption. Figure 8 shows a plot of Qe versus ln Ce, which enables the determination of the isotherm constants KT and β. The values of KT, β and correlation coefficient, R2 for Tempkin isotherm model are given in Table 1 [28,29].
| Freundlich isotherm | |||
| Adsorbent | 1/n | KF (mg g-1) | R2 |
| Moringa oleifera | 0.439 | 9.015 | 0.963 |
| Langmuir isotherm | |||
| Adsorbent | Qm (mg g-1) | KL (L mg-1) | R2 |
| Moringaoleifera | 11.36 | 0.88 | 0.9475 |
| Tempkin isotherm | |||
| Adsorbent | β | KT (mg L-1) | R2 |
| Moringaoleifera | 5.3598 | 4.829 | 0.9349 |
Table 1: Isotherm parameters for the adsorption of total iron at 30°C.
Adsorption kinetics
In order to investigate the mechanism of adsorption onto adsorbent, two kinetic models were studied: Lagergren’s first-order, Pseudo-second-order.
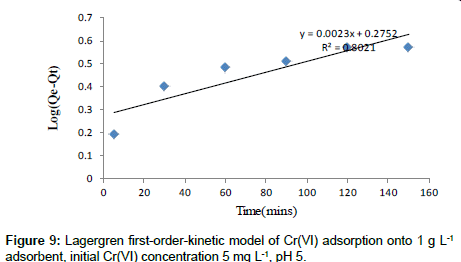
Lagergren’s first-order kinetic model: The pseudo-first-order kinetic model of Lagergren is more suitable for lower concentration of solute and its linear form is:
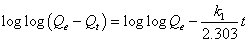 (7)
(7)
Approximately linear fits were observed for all pH and concentration indicating that sorption reaction can be approximate to first order reversible reaction model. Where, Qt (mg g-1) is the amount of adsorbate adsorbed at time t (min); Qe (mg g-1) is the adsorption capacity in the equilibrium; k1 (min-1) is the rate constant of pseudofirst- order model.
The values of k1 and Qe for Moringa oleifera adsorbents were determined from the plot of log (Qe-Qt) vs. time (Figure 9) [30,31].
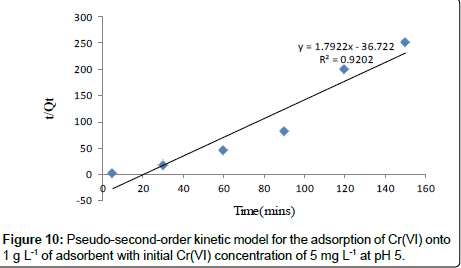
Pseudo-second-order kinetic model: Adsorption kinetics was explained by the pseudo-second-order model expressed as following linear equation:
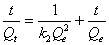 (8)
(8)
Where, k2 is the second order rate constant (g mg-1 min-1). The values of k2 for removal of chromium by Moringa oleifera adsorbents were calculated from the slopes of the respective linear plots of t/ Qt vs.t (Figure 10). The correlation coefficients, R2 were 0.920 for Moringa oleifera respectively suggest a strong relationship between the parameters and also explain that the process follows pseudo second order kinetics. Pseudo second order model better fitted to pseudo second order model [32].
Scanning electron microscope
The main features of the SEM are an electron source which provides the electrons that interact with the material to be examined an arrangement of metal apertures, magnetic lenses and scanning coils or deflectors plates that confines focuses and turns the beam of electrons into a thin and focused monochromatic beam which is accelerated towards the sample and which irradiates the specimen in a raster fashion. Moringa oleifera as such seed powder very fine particles size to the order of a millimeter or less and that there are pores within the particles of varying size. Using single Moringa oleifera as a coagulant the flocks formed with porous structure. After adsorption of Moringa oleifera seed powder chainlike and spherical structure confirms Cr(VI) onto the Moringa oleifera seed powder is shown in Figure 11.
Conclusions
In order to investigate Moringa oleifera is an effective adsorbent for the removal of Cr(VI) from aqueous solution. The adsorption is metal ion concentration is pH dependent. Batch experiments were conducted to study the effect of time, pH, temperature, initial concentration, adsorbent dosage and kinetic data showed good correlation with pseudo second order model. Maximum chromium removal 85% achieved at 120 min contact time, pH and 0.5 g of adsorbent dosage. Chemical treatment method used for the coagulation experiments it produces a secondary sludge. Natural coagulants are environmentally friendly material for removal of chromium from aqueous solution. The sludge left over after treatment it is used as biofertilizer or biocompost. The surface morphology structure of before and after adsorption of chromium(VI) were determined.
References
- Muthuraman G,Sasikala S (2014) Removal of Turbidity from drinking water using natural coagulants. Int J of indus and EngChem 20: 1727-1731.
- Muthuraman G,Sasikala S (2013) Proteins from natural coagulant for potential application of Turbidity removal in water. Int JEng and Innova Tech (IJEIT)3:278-283.
- Hong S,Kim S,Bae C (2009) Efficiency of enhanced coagulation for removal of NOM and for adsorbability of NOM on GAC. Desalination Water Treat 2: 89-94.
- Muyibi SA, Okufu CA (2007) Coagulation of low turbidity surface water with Moringa oleifera seeds. Int J Environ stud 48:263-273.
- 5.Muthuraman G,Sasikala S (2014) Reduction of Chemical oxygen demand by stabilization of pond water by various activated carbon. IntJChemtechresearch7:2924-2928.
- Muthuraman G,Sasikala S (2014) Reduction of Chemical oxygen demand by stabilization of pond water by various activated carbon. IntJChemtechresearch7:2924-2928.
- Ndabigengesere A, Narasiah KS,Talbot BG (1995) Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Wat Res 9:703-710.
- Muyibi SA,Evison LM (1995) Moringa oleifera seeds for softening hard water. Wat Res29:1099-1105.
- EmanAli N, Suleyman A, Muyibi A, Hamzah M, Salleh, et al. (2009) Moringaoliefera Seeds as a Natural Coagulant for Water Treatment, Thirteenth International Water Technology Conference, IWTC, Hurghada, Egypt. 13:163-168.
- Nasu A, Yamaguchi S, Sekine T (1997)Solvent extraction of copper(i) and (ii) as thiocyanate complexes with tetrabutylammonium ions into chloroform and with trioctylphosphine oxide into hexane. Anal Sci13:903-911.
- Santana CR,Pereira DF, Sousa SCSN, Cavalcanti EB, Silva GF(2010) Evaluation of the Process of Coagulation/Flocculation of Produced Water using Moringa oleifera Lam. as Natural Coagulant. Brazilian Journal of Petroleum and Gas 4:111-117.
- Soylak M, Erdogan ND (2006)Copper(ii)-rubeanic acid coprecipitation system for separation-preconcentration of trace metal ions in environmental samples for their flame atomic absorption spectrometric determinations. J Hazard Mater137:1035-1041.
- Ryoma Bun E,Kawasaki N,OgataF, Nakamura T,Aochi K, et al. (2006) Removal of lead and iron ions by vegetable bio mass in drinking water. J of oleo science 55: 423-427.
- Tao GH, Fang Z (1998)Dual stage preconcentration system for flame atomic absorption spectrometry using flow injection on-line ion-exchange followed by solvent extraction.J AnalChem360:156-160.
- Berbenni P, Pollice A, Canziani R, Stabile L, Nobili F (2000) Removal of iron and manganese from hydrocarbon contaminated ground waters. BioresourTechn74:109-114.
- Okuda T, Baes AU, Nishijima W, Okada M (1999) Improvement of Extraction Method of Coagulation Active Components from Moringa Oleifera Seed. Wat Res 33: 3373-3378.
- Ellis D, Bouchard C,Lantagne G (2000) Removal of iron and manganese from ground water by oxidation and microfiltration. Desalination 130: 255-264.
- Heredia JB, Martín JS (2008) Removal of Sodium Lauryl Sulphate by Coagulation/Flocculation with Moringa oleifera Seed Extract. J Hazard Mater164:713-719.
- Munter R,Ojaste H,Sutt J (2005) Complexed iron removal from ground water. Journal of EnvironmentEng131:1014-1020.
- Zalina O, Subhash B, Abdul LA (2008) Influence of the Settleability Parameters for Palm Oil Mill Effluent (POME) Pretreatment by Using Moringa oleifera Seeds as an Environmental Friendly Coagulant. International Conference on Environment, Malaysia.pp: 1-9.
- Oluduro AO,Aderiye BI (2007) Impact of Moringa oleifera Seed Extract on the Physicochemical Properties of Surface and Underground Water. International Journal of Biological Chemistry 1: 244-249.
- Muhammad RF, Nor WahidatulAzura ZN, Pang CP, HamidinN (2011) Mechanism of Turbidity and Hardness Removal in Hard Water Sources by using Moringa oleifera. Journal of Applied Sciences 11: 2947-2953.
- Reza Marandi, SeyedehMarjan BS (2011) Removal of Orange 7 Dye from Wastewater Used by Natural Adsorbent of Moringa oleifera Seeds. American Journal of Environmental Engineering 1: 1-9.
- Regina de Moreira FPM,Madeira VS,Jose HJ, Humeres E (2010) Removal of iron from water using adsorbent carbon. Separation science and Technology 39:271-285.
- Aziz HA (2004) Physicochemical removal of iron from semiaerobic landfill leachable by limestone filter. Waste Manag 24:353-358.
- Killedar DJ,Bhargava DS (1990) Feasibility of fluoride adsorption on fishbone charcoal. J InstEng (India), Environ EngDiv 70:47-49.
- Islam M,Patel RK (2006) Evaluation of removal efficiency of fluoride from aqueous solution using quick lime. J HazardMat 143: 303-310.
- Inbaraj BS,Sulochana N (2002) Basic dye adsorption on a low carbonaceous sorbent kinetics and equilibrium studies. Ind J ChemTechnol9: 201-208.
- Gopal V,Elango KP(2007) Equilibrium,Kinetic and thermodyanamics studies of adsorption of fluoride into plaster of paris. J Hazard Mater 141: 98-105.
- Krishna Veni V,Ravindhranath K (2012) Removal of chromium (VI) from polluted waters using powders of leaves or their ashes of some herbal plants. Journal of Experimental Sciences 3: 1-9.
- Allen SJ, Brown PA (1995) Isotherm analyses for single component and multi-component metal sorption onto lignite. JChem TechBiotechnol 62:17-24.
- Namasivayam C,Yamuna RT (1992) Removal of congo red from aqueous solutions by biogas waste slurry. J Chem Tech Biotech 53: 153-157.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16547
- [From(publication date):
October-2015 - Apr 06, 2025] - Breakdown by view type
- HTML page views : 11542
- PDF downloads : 5005