Research Article Open Access
Biodegradation of Coal Minerals by Gluconic Acid and its Effect on the Stacking Structure of Carbon: An Investigation
| Manoj B* | |
| Department of Physics, Christ University, Hosur Road, Bangalore-29 Karnataka, India | |
| Corresponding Author : | Manoj B Department of Physics Christ University Hosur Road Bangalore-29 Karnataka, India Tel: +91 80 4012 9340 E-mail: manoj.b@ christuniversity.in |
| Received: June 26, 2015 Accepted:July 15, 2015 Published:July 16, 2015 | |
| Citation:Manoj B (2015) Biodegradation of Coal Minerals by Gluconic Acid and its Effect on the Stacking Structure of Carbon: An Investigation. J Bioremed Biodeg 6:306. doi:10.4172/2155-6199.1000306 | |
| Copyright: ©2015 Manoj B. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Leaching of minerals (Silicates, Calcium, Aluminum) from bituminous coal by gluconic acid of varying concentration was carried out and the chemical mechanism was studied. Stacking structural parameters of the coal like aromaticity, rank, number of aromatic layers, carbon atoms per aromatic lamellae, stacking height and lattice spacing were determined using X-ray analysis. An ordering of lattice was noticed with gluconic acid leaching and is maximum for 20% and 40% concentration. The number of lyers and average number of carbon atoms per lamellae are found to be varying from 6-7 and 11-17 respectively. The mineral content (Al and Si) shows systematic decrease with increase in concentration of leachant. The optimum demineralization is noticed for the successively leached coal sample with glucoinc acid and mild Hydrofluoric acid.
| Keywords |
| Organic acid leaching; Bituminous coal; Minerals; Stacking structure; Graphite layers |
| Introduction |
| It is a known fact that coal constitute a considerable portion of the global fossil fuel reserve. A continued demand and supply of this resource generate vast quantities of spoil and low grade waste. Despite the discoveries of many microorganisms capable of lignite, lignin and humic acid breakdown, large scale bioremediation technologies for the beneficiation of low grade coal have unfortunately not yet been realized. Coal bio-solubilization technology has the potential to elevate low rank coal to either as a clean, cost-effective energy feedstock or a source of complex aromatic compounds for bio-catalytic conversion to value-added products. In recent past, the application of biotechnology in monitoring and removing metal pollution has triggered tremendous interest. An alternative process is bio-sorption, which utilizes various materials of biological origin, such as bacteria, fungi, yeast, algae, etc. They own metal-sequestering property and can be used to decrease the concentration of heavy metal ions from ppm to ppb level. It can effectually and quickly sequester dissolved metal ions out of complex molecule and is ideal for the treatment of high volume and low concentration complex industrial waste [1,2]. Living microorganisms have the ability to accumulate on metal elements and is considered from the toxicological point of view. In the present decade, extensive research is being carried out on the bio-sorption phenomena, especially in the removal of metal ions [2-5]. Fungi are large and diverse groups of eukaryotic microorganisms, of which three groups have paramount importance: molds, yeast and mushrooms. Filamentous fungi and yeast are able to bind metallic elements and can affect fermentation process. Fungi like Penicillium spp and A. niger are widely used for the elimination of heavy metal ions and radio-nuclides from aqueous solutions. A. niger is also ecologically important in biodegradation of toxic chemicals and bioconversion of waste water sludge. As it secretes carboxylic acids, A. niger can be used to bioleach metals from mining ores. |
| Organic acids may affect mineral weathering rates by at least 3 mechanisms: by changing the dissolution rate far from equilibrium through decreasing solution pH or through forming complexes with cations at the mineral surface or affecting the saturation state of the solution with respect to the mineral [5-10]. Under favourable conditions, the microorganism secretes organic acids which have the ability to degrade the coal minerals in an eco-friendly manner [11].Use of mineral acids in demineralization not only modifies the surface morphology and deteriorates the carbon structure, but also reduces the calorific value. These acids have strong oxidising power and the safe disposal of the spent liquid is a major environmental concern. For commercial utility of coal bio-demineralization, fungal leaching is an ideal eco-friendly method. But the secretion of carboxylic acid takes longer time with minimal output. In order to overcome such drawbacks, some mild organic leachants are applied directly for deashing coal. In this study, efficiency of organic acid such as gluconic acid on solubilizing silicate, aluminates and calcites mineral were discussed. |
| Materials and Methods |
| Sub-bituminous coal was air-dried and ground to the particle size <75 μm, of which 50 g was treated by employing carboxylic acids like gluconic acid (40%, 20%,10% and 5%) separately in a 500 ml teflon beaker for 24 h at room temperature (27°C). The sample was recovered from the respective organic acid solution by filtration using a polypropylene funnel. It was washed repeatedly in distilled water to remove the acid contents and finally dried in an oven at 80°C. The quantification of minerals in virgin and bioleached coal samples were carried out using a SEM (JEOL model JED-2300). The XRD pattern was recorded by a Bruker AXS D8 Advance X-ray powder diffractometer. Powdered samples were scanned from 4-70° in 2θ range with 0.020°step intervals and 2 s/ step counter time. The structural parameters are elucidated from the XRD analysis of the sample using the following equations (1-4) |
| The aromaticity (fa) of coal (ratio of carbon atoms in aliphatic chain to aromatic rings) |
| fa=A002/ (A002+Aϒ) (1) |
| where A is the integrated under the respective peak |
| Coal rank=I26/I20 (2) |
| Stacking height Lc=0.89λ/Bccos θc (3) |
| where λ is the wavelength of X-ray used and Bc is half width of the (002) peak while θc is the scattering angle of (002) peak in radian. |
| The number of layers and average number carbon atoms per aromatic lamellae can be estimated by the formula |
| N=Lc+ d002/d002 and n=0.32 N2 (4) |
| Result and Discussion |
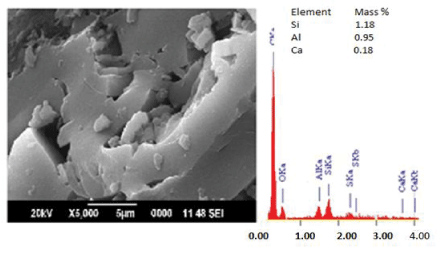
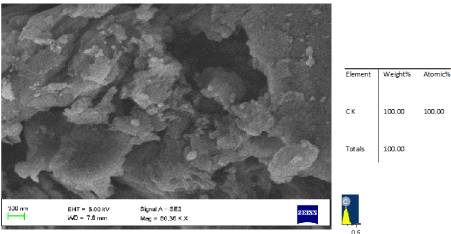
| The SEM-EDS analysis was performed on the virgin and gluconic acid solubilized products in order to monitor the change in mineral content and surface morphology. The micrographs (Figures 1-6) revealed coal structure is composed of homogeneously distributed network of small mineral crystallites. Many fissures, cleats, cracks and veins were also observed. The luminosity is due to the presence of aluminum, potassium and sodium, while the dark regions indicate chalcophiles [7-10]. Randomly distributed etch pits, layers, islands, hills and valleys could also be noticed,which might have resulted from the calcinations of dolomite and calcites or their assemblages, owing to thermal shock during metamorphism [9-12]. It is evident that, the solubilized coal contains large proportions of silicates, calcium carbonates and dolomite, as well astraces of aluminum and sulphur. The elemental composition quantified by EDS (Si-1.18 wt%; Al-0.95 wt% and Ca-0.18 wt%) indicated Si and Al as major minerals in the virgin sub-bituminous coal. The bright particles observed on the micrograph are due to bassanite and kaolinite. The SEM-EDS profile of the coal sample treated with 40% gluconic acid. |
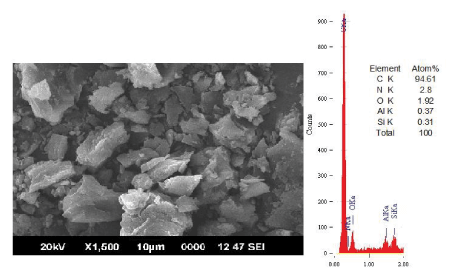
| Figure 2 showed that leaching caused changes in the morphology of coal ( C=94.61 wt%, N=2.8 wt%, O=1.92 wt% Al=0.37 wt% and Si=0.31 wt%). With gluconic acid treatment, calcium minerals were removed with the formation of calcium gluconate. |
| 2(C6H12O7.) → (C12H22O14)2- + 2 H+ |
| C12H22O14 + Ca2+ → C22H22CaO14 (Calcium gluconate) |
| The experiments performed using various concentration of carboxylic acids and the results of SEM-EDS analysis is presented in Table 1. |
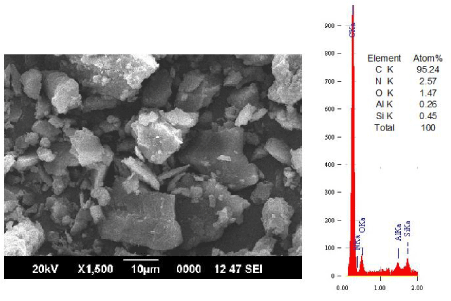
| The content of carbon is increased significantly above 93.% in all the cases. The mineral content shows a systematic decrease with increase in concentration. A drastic reduction in the nitrogen and oxygen content with increase in concentration of the leachant was noticed. This implies that there is no oxidation happened to the coal matrix during leaching. The silicate content is changed from 1.10 wt% to 0.30 wt%. whil e the aluminates reduced to 0.27 wt% with leaching. |
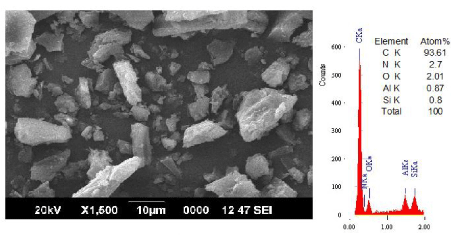
| The gluconic acid treated sample (40%) is further treated with hydrofluoric acid (10%) to demineralize the bound minerals. The analysis confirmed total removal of aluminates and silicates with the formation of fluro-silicates and aluminates. The oxygen and nitrogen content is totally eliminated along with minerals. |
| The SEM-EDS analysis of the coal sample treated with gluconic acid and HF is presented in Figure 6. The micrograph shows the morphology of nano-graphene layers. The EDS analysis of the surface indicates only carbon in the form of flakes |
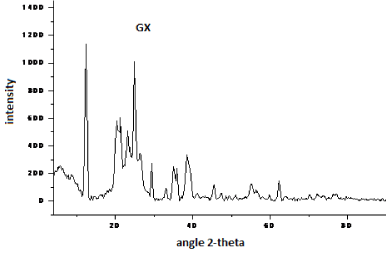
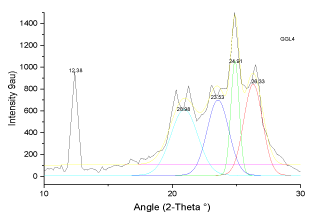
| The X-ray diffractograms of pure graphite and bioleached samples are depicted in Figures 7-12. The study on X-ray scattering from coal has paramount importance, as it enables quantification of low and high temperature ash making mineral. The diffraction profiles were recorded using a BRUKER D8 advance powder diffractometer (XRD) with nickel filtered CuKα radiation (λ=1.5406 Å). The patterns were examined over the 2-theta range of 5-90�?�?, with a scan step of 0.02�?�? s-1The peaks observed at 12.4, 20.5 and 33.3�?�?are assigned to kaolinite (Al2Si2O5(OH)4), while, that at 29.3�?�?is because of the presence of dolomite in the samples [9-15]. Except for the intense sharp spikes corresponding to inorganic components such as kaolinite, pyrite, quartz, crystoballite and mullite, the strong diffraction maxima at 25.8�?�? is due to crystalline carbon in coal samples. The weak peak at 43° is ascribed to (101) plane reflection of graphite [9,15]. This is due to the random layer lattice structure of crystallites in coal [12-15]. The profiles exhibited strong diffraction peaks, suggesting the crystallinity of Indian coals. |
| The X-ray diffraction profiles for demineralized coal samples (Figures 7-12) exhibited intense background, confirming highly disordered amorphous carbon. The X-ray spectrum is deconvoluted by origin pro 2015 software to identify the different type of carbon structure present in the sample. Upon deconvolution all the samples shows four peaks, which is attributed to the carbon in different hybridization. |
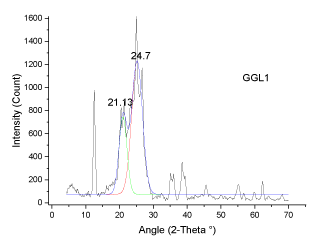
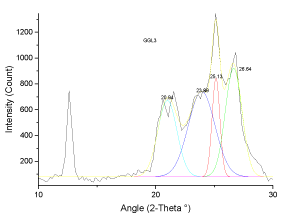
| The deconvoluted X-ray spectrum shows four major peaks at ~21�?�?, 23.89�?�?, ~25�?�? and ~26.5�?�?. The peak at 25�?�? and 23.89�?�? is attributed to the graphitic peak and the associated defect carbon in it. The peak at 21�?�? is originating from the amorphous carbon in the coal matrix while the inherent minerals shows the prominent band at ~26.5�?�? |
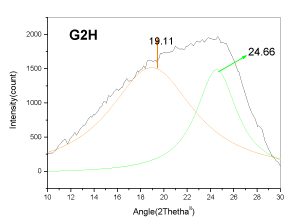
| The XRD profile of the HF and Gluconic acid sample (G2H) shows a different profile compared to the other samples. There are only two peaks at ~20 and ~25�?�?. The existence of crystallites in coal structure was identified by the (002) and (110) reflections in the XRD spectrum [10- 13]. There is absence of mineral structure after leaching. This finding is in support with the SEM-EDS analysis |
| These observations conclude that, the crystallites in all coal samples possess intermediate structures between graphite and amorphous state, the so called turbostratic or random layer lattice structure [11]. All the samples exhibits two defined peaks at ~25° (π-band) and 20° (γ-band) and has more of graphitic or ordered structure.he intensity ratio (I20/ I26) of γ to π-band is a measure of imperfection in amorphous carbon [10-15]. The lower the ratio, higher the ordering of carbon layers in coal sample and it approaches graphitic nature. The calculated structural parameters are presented in Table 2. |
| Stacking structural parameters of the solubilized coal such as aromaticity, rank, number of carbon atoms per aromatic lamellae, stacking height and lattice spacing were determined using X-ray diffraction analysis. It is observed that there is ordering of the lattic stacking with leaching. The aromaticity is found to be around 0.74 for the sample which is leached with 20% gluconic acid. The rank is also highest when leached 20% and 40% gluconic acid. The interlayer spacing is very close to that of graphite layers (0.343nm). Number of aromatic layers and average number of carbon atoms per aromatic lamellae is also found to vary between 6-7 and 11-17 respectively. The values of stacking parameters are in very close agreement to that of HF leached coal sample. |
| Conclusions |
| Coal biodegradation is a naturally complex process, which appears to be driven by extracellular enzymes in the presence of various chelators released by different fungi. Despite slow conversion rates in the biological breakdown of coal, optimization of the process on a large scale develop the technology for remediation of low rank coals. The calcites mineral content in coal samples was completely removed by leaching with gluconic acid. The intensity ratio (I26/I20), a measure of disorder in amorphous carbon, was found to be 1.80 and 1.82 when leached with gluconic acid of concentration 20% and 40% respectively. The lateral size along the c-axis (Lc) was varied from 2.06 to 1.90 nm as the concentration of gluconic acid varied from 10% to 40%. Gluconic acid (40% and 30%) was able to remove minerals efficiently, than other concentration as is evident from the XRD studies and EDS analysis. The interlayer spacing of sample leached with 10, 20 and 40% gluconic acid leached sample were found to be 0.344 nm, which is near to that of ordered graphite (0.335 nm). It is conclude that with mild leachant like gluconic acid, there is ordering of the stacking parameters of amorphous carbon in coal. There is a systematic elimination of mineral content in the coal matrix with optimum removal during the combined leaching of mineral acid and gluconic acid. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 | Figure 8 |
 |
 |
 |
 |
| Figure 9 | Figure 10 | Figure 11 | Figure 12 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 13810
- [From(publication date):
July-2015 - Jun 29, 2024] - Breakdown by view type
- HTML page views : 9431
- PDF downloads : 4379
