Research Article Open Access
Antigenic Screening for Helicobacter pylori in Stool of Patients Infected with Human Immunodeficiency Virus from a Tertiary Care Hospital
Bineeta Kashyap*, Kavita Gupta, Purbasha Bera and Iqbal R KaurDepartment of Microbiology, University College of Medical Sciences (UCMS) & Guru Teg Bahadur (GTB) Hospital, Delhi, India
- *Corresponding Author:
- Bineeta Kashyap
Flat no. C-402, Vimal CGHS Ltd.
Plot-3, Sector-12, Dwarka, New Delhi, India
Tel: +91-11-22582972-74
E-mail: dr_bineetakashyap@yahoo.co.in
Received date: September 25, 2015; Accepted date: November 13, 2015; Published date: November 24, 2015
Citation: Kashyap B, Gupta K, Bera P, Kaur IR (2015) Antigenic Screening for Helicobacter pylori in Stool of Patients Infected with Human Immunodeficiency Virus from a Tertiary Care Hospital. J Emerg Infect Dis 1:101. doi:10.4172/2472-4998.1000101
Copyright: © 2015 Kashyap B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Disease and Pathology
Abstract
Introduction: Human immunodeficiency virus (HIV) infected patients frequently have opportunistic gastrointestinal presentation. Co-morbidity of Helicobacter pylori infection in HIV patients is not well defined. The aim of the study is to evaluate, whether antigenic screening of stool is a reliable test to identify new Helicobacter pylori infections in HIV infected population.
Materials and Methods: Stool samples from 50 HIV reactive subjects (cases) 16 years-65 years age group presenting with diarrhea were screened for the presence of H. pylori antigen along with equal number of HIV nonreactive (control) subjects presenting with diarrhea by ELISA.
Results: H. pylori antigen was detected in 10% of cases while none of the controls was found to be positive for the presence of H. pylori antigen (p<0.05). No significant correlation was found between risk of acquisition of infection and age or sex.
Conclusion: More emphasis should be given on the screening of H. pylori infection in immunocompromised population to clearly define the management protocol in such patients.
Keywords
Helicobacter pylori; HIV; Stool antigen; Screening; ELISA
Introduction
Helicobacter pylori (H. pylori ), is a causative agent for gastric damage and has been linked to many diseases, including gastritis, peptic ulcer, and gastric malignancies, since its identification in 1982 [1]. Though half of the world’s population is infected with H. pylori , only approximately 30 percent of the infected people are symptomatic with more than 70 percent of them remaining asymptomatic [2]. Seroepidemiological research studies are consistent with the hypothesis that H. pylori infection is mainly acquired in early childhood and that primary acquisition or reinfection after successful eradication in adults occur less frequently with an annual incidence of 6%-14% in developing countries [2]. Though H. pylori is one the human pathogens with highest prevalence all over the world; the mode of infection, geographic variation, associated risk factors, host immune response, genetic heterogeneity and polyclonal infection pertaining to the agent still are subject to debate [3].
H. pylori infection can be diagnosed by a battery of invasive and non-invasive tests each with inherent advantages and disadvantages. While the invasive tests like culture or histopathology of biopsy samples are liable to sampling error due to the patchy or low level infection; the non-invasive tests like serology on the other hand are unreliable due to lack of validation or persistence of antibodies. The stool antigen test, used to detect the presence of H. pylori antigens shed in the feces, has reported sensitivity and specificity similar to that of the 13C-urea breath test [2].
Human immunodeficiency virus (HIV) infected patients with acquired immunodeficiency syndrome (AIDS) frequently have opportunistic gastrointestinal presentation as HIV infection profoundly impacts gut function [4]. The co-morbidity of H. pylori infection in HIV patients is not well defined and the role of H. pylori in gastro duodenal lesions might be different between the general population and AIDS patients [5,6]. Previous studies done on prevalence of H. pylori in association with HIV infection have suggested that cell mediate immune deficiency does not appear to increase the risk of infection with H. pylori [7]. Others however suggest host’s immune competence to be a critical issue as interactions between the immune response, gastric physiology and host repair mechanism seem to contribute significantly in dictating the disease outcome [5,8,9]. Hence there have been diverging estimates of prevalence of H. pylori infection in association with HIV AIDS. Moreover most of the estimates have been done using antibody prevalence which becomes unreliable in context to a developing country where a big majority of individuals acquire the infection in early childhood. The aim of the study is to evaluate, whether antigenic screening of stool is a reliable test to identify new H. Pylori infections in HIV infected population.
Materials and Methods
A prospective cross sectional study was done in the immunology section of microbiology, University College of Medical Sciences (UCMS) and Guru Teg Bahadur (GTB) Hospital, Delhi. Ethical approval was sought from the Institutional Ethical Committee and informed consent was taken from all the study participants.
Fifty HIV reactive patients attending the ART (anti-retroviral treatment) presenting with diarrhea were included as cases in the study. Fifty age and sex matched HIV nonreactive individuals presenting with diarrhea served as controls. The stool samples from 50 cases and 50 controls were screened for the presence of Helicobacter pylori antigen. Exclusion criteria included age below 16 years or above 65 years, other serious medical problems, or previous treatment for H. pylori infection.
Stool samples obtained from each participant were collected in airtight containers and were transported to the laboratory and stored at -20°C before analysis by HP Ag Enzyme Immunoassay for the qualitative determination of H. pylori antigen in human stools (DIA.PRO Diagnostic Bioprobes, Italy).
Direct saline and iodine wet mounts were prepared from all stool samples and examined for the presence of cyst and ova of parasites. Formal ether concentration was performed for all negative samples and wet mount examined again after concentration. Screening for coccidian parasites was done using modified acid fast staining in all HIV sero positive patients to rule out parasitic cause of diarrhoea. To rule out bacterial cause culture was done on Xylose, Lysine, Deoxycholate Agar.
All the stool samples received were screened for the presence of any ova or cyst to rule out any parasitic infection. The HP Ag is a sandwich ELISA where microtitre plates are coated with affinity mouse monoclonal antibody directed to the most specific H. pylori antigen. The test was performed as per manufacturer’s instructions and the diagnostic sensitivity and specificity of the test kit was 95% and 96% respectively as claimed by the manufacturer. The samples were removed from the freezer and thawed and thoroughly mixed so that the probable antigens could locate all over the stool sample constantly. Stool sample approximately the size of a pea was added to 200 μl of the triple buffers, i.e., 0.05 M saline phosphate buffer, saline phosphate buffer containing 0.1% triton X-100, and 1.5 M glycine buffer with pH=7.2 which was used as a diluent. It was mixed thoroughly using a vortex mixer. Subsequently, the samples were centrifuged at 5000 rpm for 10 minutes. The supernatant was transferred to a 1.5 ml Eppendorf tube that was then used for the ELISA test as per kit instructions. Positive and negative controls were used provided with the kit. The results were interpreted as follows: OD450<0.140 was negative, OD450>0.160 was positive and the value in between was equivocal. Any equivocal result was repeated. The analytical sensitivity of the assay was better than 0.05 μg/ml when the limit of dilution was considered mean OD 450 nm CAL 0 μg/ml+5 SD.
Data were analyzed using the software SPSS. Chi square test was used to compare the result among the different groups. Significance was accepted at p<0.05.
Results
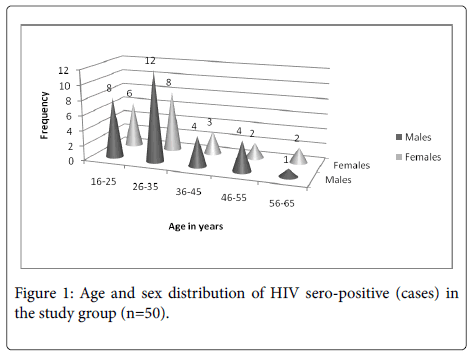
The study group consisted patients presenting with diarrhea out of which fifty were HIV reactive (cases) and fifty age and sex matched HIV non-reactive (controls). Among the cases majority of the patients were in age groups 16 years-25 years and 26 years-35 years. The mean age of the HIV infected patients was 32.9 years (range 16 years-65 years) and 58% of them (29/50) were males. The detailed age and sex distribution of the cases is shown in Figure 1.
Out of the fifty stool samples received from the HIV sero-positive cases 26 were formed stool, 21 semi formed and 3 were of liquid watery consistency. All the samples were screened for the presence of any ova or cyst. Only one sample showed the presence of fertilized egg of Ascaris lumbricoids. H. pylori antigen was detected in 5 (5/50,10%) HIV sero-positive subjects (cases) while none of the subjects among the HIV sero-negative group (controls) was found to be positive for the presence of H. pylori antigen (Table 1).
| Study group | Helicobacter pylori Ag in Stool | Total | Percentage (%) | P value | |
|---|---|---|---|---|---|
| Present | Absent | ||||
| HIV sero-positive (Cases n=50) | 5 | 45 | 50 | 10 | < 0.05 |
| HIV sero-negative (Controls n=50) | 0 | 50 | 50 | 0 | |
| Total | 5 | 95 | 100 | 5 | |
Table 1: Correlation of Helicobacter pylori Ag in stool with HIV sero prevalence in the study group (n=100).
None of the positive samples for H. pylori showed any bacterial or parasitic co infection. Figure 2 shows the age wise positivity for H.pylori Ag in the stool of HIV seropositive cases with the highest prevalence in 26-35 years of age group (60%) followed by 16 years-25 years (20%) and 36 years-45 years (20%), while it was absent in subjects above 45 years of age. Among the five H. pylori antigen positive subjects, 4 (80%) were females (p>0.05).
Discussion
Helicobacter pylorus resides in the antral region of the human stomach and has not been consistently isolated from any other niche, and hence the mechanism of its colonization of the human stomach remains largely unknown. The growing attention given to this previously underestimated clinically relevant organism is largely due to the evident geographic variation of this infection probably due to combination of various factors like age, gender, genetic predisposition, ethnicity, epidemiology and socioeconomic status. Marked difference exists in the prevalence of H. pylori between developed and developing countries. Whereas in the developed world with high socio-economic status, the prevalence of H. pylori ranges between 15% and 54% in the general population, the prevalence s high ranging from around 70% upto 85% in the developing countries [3,7,10].
One of the most controversial aspect in the study of this pathogen is the mode of transmission; fecal-oral route being the most likely portal of entry. Though culture of H. pylori from the gastric secretion is frequently attempted, isolation is difficult from stool or the oral cavity because of the diverse, abundant resident microbiota. Malnourishment and short fecal transit time have been suggested to be reasons behind the exceptional isolation of H. pylori from stool samples [11].
Since H. pylori infection elicits both a local mucosal and a systemic antibody response, antibody assays have been used widely in epidemiological studies, to determine the prevalence or incidence of this infection. However accuracy of these serological tests depends upon proper validation due to the considerable variation in individuals’ antibody response and failure of these assays to determine eradication, measure reinfection rates or identify cases of primary infection [2].
Since the advent of high active antiretroviral therapy (HAART) and more so because the gut-associated lymphoid tissue (GALT) represents the largest reservoir of HIV in the body gastrointestinal tract involvement in HIV seropositive individuals causes considerable morbidity due to multiple etiologies. Despite H. pylori Fialho et al. have reported the overall prevalence of H. pylori infection to be significantly lower in HIV-infected patients when compared with the controls (37.2% versus 75.2%) and that the prevalence did not increase with age with the infection prevalence in the oldest group being the same between HIV-positive and HIV negative patients.Unlike this report our study reports a significantly higher prevalence of H. pylori infection in HIV-infected patients when compared with the controls (10% versus nil). Also the association between the H. pylori status and the age or gender among the HIV positive patients was not significant in our study in concordance with the above study [12]. All five H. pylori antigen positive HIV subjects were form low socioeconomic status.
Studies from countries like Taiwan and China with high H. pylori infection prevalence have demonstrated a lower H. pylori infection prevalence (17.3% and 22.1%, respectively) in HIV-infected than in non- infected (63.5% and 44.8%, respectively) patients [13,14]. On the other hand other studies including one from India showed relatively similar H. pylori infection prevalence in HIV infected and noninfected patients [4,6,15]. Various hypotheses given for a lower prevalence of H. pylori in HIV positive patients compared to the noninfected ones include competitive inhibition by opportunistic pathogens, intragastric environment modification by previous use of proton pump inhibitors, prophylactic use of antibiotics for opportunistic infections in HIV AIDS and low CD4 count or hypochlorhydria that has been described in HIV leading to altered gastric environment for H. pylori or predisposition to overgrowth by other bacteria [12,14].
Variability in the prevalence of H. pylori infection from one region to another is primarily due to the fact that the prevalence of this infection is related to the socioeconomic conditions or education status of the population under study. The use of an active antigen method to investigate the colonization of H. pylori in this study could be responsible for a relatively higher prevalence of this infection in HIV infected population. A positive result in such a test is evidence of a current infection and not the possibility of a previous infection that is suggested by a serological test done for antibody detection. Moreover in a participant with severe immunodeficiency a test based on antibody detection could eventually show a false negative result due to an inadequate immune response. While various studies have reported high sensitivity and specificity of the antigen test in non-HIV-infected populations, [16,17] others have recommended it for screening in HIV-infected population [5]. A study done to screen the presence of H. pylori in stool of HIV infected patients by amplification of two conserve genes of H. pylori reported high prevalence of this bacterium in these patients and also suggested that H. pylori is present in gastric of HIV-infected patients but cannot reveal its clinical symptoms and cannot develop disease [18].
After the advent of potent antiviral therapy (HAART), an increase in the incidence of H. pylori infection and its complications in HIVinfected patients has been shown [19]. Since then, an extensive study on its epidemiology suggests that not all humans, more so those being immunocompromised, are equally at risk of infection by this gut pathogen. More emphasis should be given on the screening of this infection in such population in order to clearly define the management and the protocols in the presence of dyspepsia in HIV-infected patients or patients with AIDS.
References
- Suerbaum S, Michetti P (2002) Helicobacter pylori Infection. N Engl J Med 347: 1175-1186.
- Logan RPH, Walker MM(2001) Epidemiology and diagnosis of Helicobacter pylori infection. British Med J323: 920-922.
- Khalifa MM, Sharaf RR, AzizRK (2010) Helicobacter pylori: a poor man’s gut pathogen? Gut Pathog2:2.
- Sud A, Ray P, BhasinDK, Wanchu A, Bambery P, et al. (2002) Helicobacter pylori in Indian HIV infected patients. Trop Gastroenterol 23: 79-81.
- Romanelli F, Smith KM, Murphy BS(2007) Does HIV infection alter the incidence or pathology of Helicobacter pylori infection? AIDS Patient Care STDS 21:908-919.
- Alimohamed F, LuleGN, Nyong’o A, Bwayo J, Rana FS (2002) Prevalence of Helicobacter pylori and endoscopic findings in HIV seropositive patients with upper gastrointestinal tract symptoms at Kenyatta national hospital, Nairobi. East Afr Med J 79: 226-231.
- Okoth FA (2002)H. Pylori: Does co-morbidity affect the prevalence? East African Med J 79: 225.
- Bamford KB, Fan X, Crowe S, Leary JF, Gourley WK, etal. (1998) Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology114:482-492.
- Moran AP, Svennerholm AM, Penn CW (2002) Pathogenesis and host response of Helicobacter pylori. Trends Microbiol 10: 545-547.
- Perez-Perez GI, Rothenbacher D, Brenner H (2004) Epidemiology of Helicobacter pylori infection. Helicobacter 9:1-6.
- Mégraud F (1995) Transmission of Helicobacter pylori: faecal-oral versus oral-oral route. Aliment PharmacolTher 9:85-91.
- Fialho ABC, Braga-Neto MB, Guerra EJC, Fialho AMN, Fernandes KC, et al. (2011) Low prevalence of H. pylori Infection in HIV Positive Patients in the Northeast of Brazil. BMC Gastroenterology 11:13.
- Chiu HM, Wu MS, Hung CC, Shun CT, Lin JT (2004) Low prevalence of Helicobacter pylori but high prevalence of cytomegalovirus associated peptic ulcer disease in AIDS patients: Comparative study of symptomatic subjects evaluated by endoscopy and CD4 counts. J GastroenterolHepatol19:423-428.
- LV FJ, Luo XL, Xin M, Jin R, Hui-Guo D, et al.(2007) A low prevalence of H pylori and endoscopic findings in HIVpositive Chinese patients with gastrointestinal symptoms. World J Gastroenterol13:5492-5496.
- Olmos M, Araya V, Pskorz E, Quesada EC, Concetti H, et al.(2004) Coinfection: Helicobacter pylori/human immunodeficiency virus. Dig Dis Sci49:1836-1839.
- Kato S, Ozawa K, Okuda M, Nakayama Y, Yoshimura N, et al. (2004) Multicenter comparison of rapid lateral flow stool antigen immunoassay and stool antigen enzyme immunoassay for the diagnosis of Helicobacter pylori infection in children. Helicobacter 9:669-673.
- Nares-Cisneros J, Jaramillo-Rodriguez Y, Martinez-Ordaz VA, Velasco-Rodriguez VM, Madero A,et al. (2007) Immunochromatographic monoclonal test for detection of Helicobacter pylori antigen in stool is useful in children from high-prevalence developing country. Helicobacter 12:354-358.
- Kafil HS, Jahromi FF, Hajikhani B, Pirayeh SN, Aghazadeh M (2011) Screening for the presence of Helicobacter pylori in stool of HIV-positive patients. J AIDS HIV Res 3: 85-87.
- Chehter EZ, Catapani WR, Margeotto FB, Germini D, Henriques AC, et al.(2014) Helicobacter pylori in the Era of Highly Active Antiretroviral Therapy (HAART): A Review. JSM Gastroenterol Hepatol 2: 1026.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15636
- [From(publication date):
March-2016 - Jul 04, 2025] - Breakdown by view type
- HTML page views : 10964
- PDF downloads : 4672