Research Article Open Access
A New Optical Sensor for Selective Monitoring of Nickel Ion Based on A Hydrazone Derivative Immobilized on the Triacetyl Cellulose Membrane
Kamal Alizadeh* and Nasim Abbasi Rad
Department of Chemistry, Lorestan University, Khorramabad, Iran
- Corresponding Author:
- Kamal Alizadeh
Department of Chemistry, Lorestan
University, Khorramabad, Iran
Tel: +986633120612;
Fax: +986633120612
E-mail: Alizadehkam@yahoo.com (or) Alizadeh.k@lu.ac.ir
Received date: May 24, 2016; Accepted date: June 10, 2016; Published date: June 17, 2016
Citation: Alizadeh K, Rad NA (2016) A New Optical Sensor for Selective Monitoring of Nickel Ion Based on A Hydrazone Derivative Immobilized on the Triacetyl Cellulose Membrane. J Anal Bioanal Tech 7:322. doi:10.4172/2155-9872.1000322
Copyright: © 2016 Alizadeh K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A new highly selective optical sensor was prepared by de-esterification of triacetyl cellulose transparent film and chemical immobilization of 1-acenaphthoquinone 1-thiosemicarbazone (L) on it. The absorbance variation of immobilized 1-acenaphthoquinone 1-thiosemicarbazone on hydrolyzed cellulose acetate film of upon addition of 1.5 × 10-5 mol L-1 aqueous solutions of Zn2+, Pb2+, K+, Cu2+, Ag+, Ni2 , Cd2+, Ca2+, CrO4 2-, Hg2+, Co2+, Mn2+, Cr3+, S2 O3 2-, Mg2+, Na+, Al3+, Tl+ and Fe3+ indicated a substantiality much larger variation for the Nickel ion in compare to other studied ions. Consequently, the new hydrazone derivative L possesses a high selectivity towards this metal ion. Influences of various experimental parameters on Ni2+ sensing, including the reaction time, the solution pH and the concentration of reagents were studied. A linear relationship was observed between the variance in membrane absorbance(â�?�?A) at 337 nm and Ni2+ concentrations in a range from 5.01 × 10-10 to 2.04 × 10-5 mol L-1 with a detection limit (3σ) of 1.00 × 10-10 mol L-1. No significant interference from 100 times concentrations of a number of potentially interfering ions was detected for the nickel ion determination. The sensor showed a good durability and short response time with no evidence of reagent leaching. The optical sensor was successfully applied to the determination of nickel in real water samples.
Keywords
Optical sensor; Nickel ion; Triacetyl cellulose membrane; Hydrazone derivative; Spectrophotometric
Introduction
In the recent years, pollution of the environment by heavy metals has received considerable attention. Nickel is a moderate toxic element compared to other transition metals. However, it is known that inhalation of nickel and its compounds can lead to serious problems, including respiratory system cancer. Moreover, nickel can cause a disorder known as nickel-eczema [1,2]. Nickel is an excellent alloying metal in steel industry and is the metal component of the enzyme urease and as such is considered to be essential to plants and some domestic animals [3]. This metal normally occurs at very low level in the environment, so sensitive methods are needed to detect it in most environmental samples. Thus, the development of simple methods for selective determination of nickel in trace amounts in different matrices is critical.
Optical sensors have found great interest in recent years as they have many uses in clinical analysis, environmental analysis, and process control [4]. Optical chemical sensors (optodes), are usually based on acid–base indicators, which can be adsorbed on the surface of support materials [5-7]. Several different support materials including lipophilic polymers optical sensors [5-8]. Covalently immobilized dyes, in contrast to the physically adsorbed or entrapped dyes, do not suffer from leaching or hysteresis and exhibit long lifetimes [8].
Optodes are simple and selective tools for the determination of heavy metal ions that have been extensively developed in recent years. Optodes are generally used in combination with inexpensive spectrophotometic or spectroflourometic techniques to provide simple and fast determination methods with enhanced selectivity and low detection limits [9-13].
Fabrication of membrane optical sensors have been reported for many cations including Ca2+, Na+, K+, Ni2+, Pb2+, Hg2+ and Cu2+ [14-22]. Use of transparent triacetyl cellulose and agarose membranes as supports for preparation of covalently immobilized optical sensors for some ions determination were reported by different laboratories. It was shown that these membranes can be easily manufactured and simply activated and functionalized with an ionophore. In construction of optical sensors, ionophores play an important role. The compounds contain some donor atoms have been frequently used as ionophores in construction of membrane sensors because of their ability to form stable complexes with transition metal ions. They produce remarkable selectivity, sensitivity and stability for a specific ion [23-26].
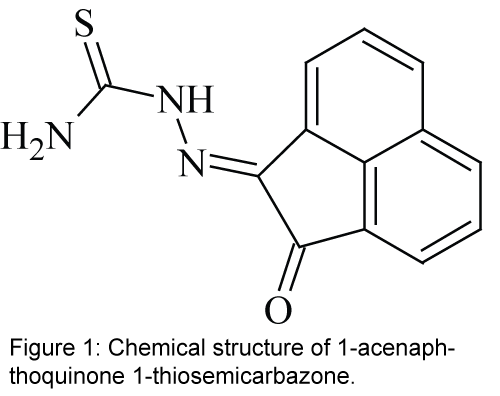
In the present study, a hydrazone derivative ligand 1-acenaphthoquinone 1-thiosemicarbazone, L (Figure 1) [27,28], is covalently immobilized on a triacetyl cellulose membrane to be used as an effective ionophore with N and O donor atoms for construction of a selective optical sensor for the spectrophotometric determination of Ni2+ in aqueous solutions. The studied compound as an ionophore is the family of hydrazone which is a kind of asymmetric Schiff’s base. Schiff's bases (also called azomethines or imines) are functional groups with the general formula of R1R2C=N-R3. Schiff’s base can be divided into two groups; symmetric and asymmetric Schiff's base. Hydrazones are the members of the asymmetric Schiff's bases. Hydrazones are a class of organic compounds with the general structure of R2C=NNR2 which are related to ketones and aldehydes by the replacement of the oxygen with NNR2 functional group. These compounds are commonly formed through the reaction of hydrazine on ketones or aldehydes [29-31].
Experimental Section
Materials and instruments
All reagents were of analytical reagent grade and were used as received. Deionized double-distilled water was used throughout and test solutions were buffered in a 0.02 mol L-1 solution of acetic acid/ sodium acetate and pH adjusted with dropwise addition of 1 mol L-1 solution of HCl or NaOH. The Schiff’s base L, with the chemical name of 1-acenaphthoquinone 1-thiosemicarbazone, was synthesized and purified using a previously reported method [27,28].
A Jenway (USA) model 3020 pH meter with a combined glass electrode was used after calibration against standard Merck buffers for pH determinations. A Shimadzu (Japan) model 1650PC double-beam spectrophotometer was used for running the electronic absorption spectra (controlled to ± 0.1°C). A home-made polyacrilamide holder was used for holding triacetyl cellulose membranes inside the quartz cells of the spectrophotometer. A totally glass Fisons (UK) double distiller was used for preparation of doubly distilled water.
Procedures
A method described elsewhere was used for the preparation of transparent triacetyl cellulose membranes. They were produced from waste photographic film tapes, which were previously treated with commercial sodium hypochlorite for several seconds in order to remove the colored gelationous layers [26,32]. The triacetyl cellulose transparent film was hydrolyzed in order to deesterify the acetyl groups and to increase the porosity of the membrane by treating the membrane into 0.10 M NaOH solution for 24 h. The films were treated with a solution of 0.007 g of the compound L, in 10 ml ethylene diamine for 2 min at ambient temperature. Afterwards, they were washed with water for the removal of ethylene diamine and the loosely trapped indicator. The prepared membranes were kept under water, when not in use. A 1 cm × 2 cm peace of the fabricated membranes sensor was cut and mounted in a polyacrylamide holder and placed inside the quartz cell of the spectrophotometer. The cell was then used as usual for the absorbance determinations. All the measurements on the transparent triacetyl cellulose membrane were performed in aqueous medium.
The recommended procedure was applied to the determination of nickel in several real water samples collected from the west of Iran, Kermanshah. The pH was adjusted to 6.0 before analysis, without any further treatment. For analysis, about 2.5 ml of the samples were transferred into a 1 cm quartz cell equipped with the membrane sensor. The absorbance’s were then measured at 337 nm and subtracted from an absorbance reading for a buffer solution at the same wavelength. The Ni2+ concentration was then derived using an ordinary calibration curve method.
Results and Discussion
Preliminary studies
In preliminary studies, we recorded the absorbance variations followed by absorbance readings at maximum wavelength of immobilized 1-acenaphthoquinone 1-thiosemicarbazone on hydrolyzed cellulose acetate into a quartz cell of the spectrometer. They occur upon addition of 1.5 × 10-5 mol L-1 aqueous solutions of Zn2+, Pb2+, K+, Cu2+, Ag+, Ni2, Cd2+, Ca2+, CrO42-, Hg2+, Co2+, Mn2+, Cr3+, S2O32-, Mg2+, Na+, Al3+, Tl+ and Fe3+ which was obtained after equilibration at pH 6. According to the shape reported in Figure 2, It should be noted that the largest variation is observed for Ni2+, whereas for the other studied ions, negligible or small variations in the absorbance maximum is observed by increasing the concentration of corresponding ions. Based on the relatively high selectivity of 1-acenaphthoquinone 1-thiosemicarbazone for Ni2+, as was concluded from its absorbance variation, the mentioned compound, L was expected to possess a high selectivity towards this metal ion.
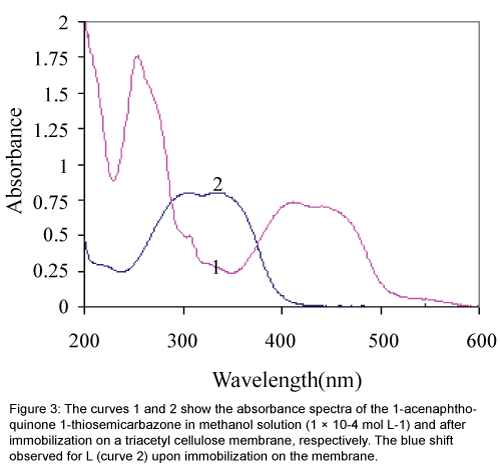
Immobilization of ligand L on a triacetyl cellulose transparent film changed, in some extent, its optical properties. The absorbance maximum of L showed a blue shift from 417 nm to about 337 nm upon the immobilization as is obvious from Figure 3. This can suggest that the structured conformation of the immobilized 1-acenaphthoquinone 1-thiosemicarbazone compound is less flat than that of its soluble analogue [26]. Furthermore, it is evident that the first region of spectra at about 200-300 nm for the membrane sensor in compare to the dissolved form of L, in methanol was disappeared too.
Figure 3: The curves 1 and 2 show the absorbance spectra of the 1-acenaphthoquinone 1-thiosemicarbazone in methanol solution (1 × 10-4 mol L-1) and after immobilization on a triacetyl cellulose membrane, respectively. The blue shift observed for L (curve 2) upon immobilization on the membrane.
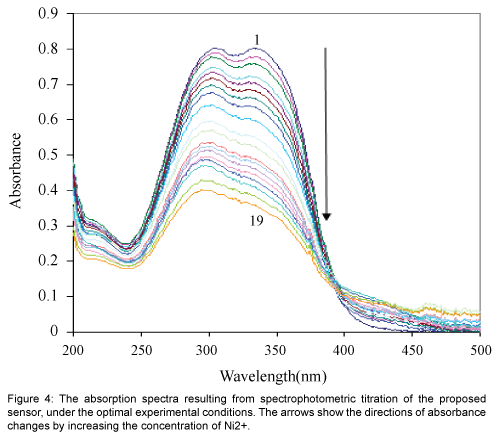
Figure 4 show the absorption spectra of immobilized 1-acenaphthoquinone 1-thiosemicarbazone on hydrolyzed cellulose acetate which was obtained after equilibration at pH 6.0 containing different concentrations of Ni2+. The spectral characteristic of this optical sensor indicate maxima at 337 nm. It is evident that the membrane absorbance at 337 nm decrease by increasing Ni2+ concentration as a result of the complex formation in the optode. During the titration, no measurable spectral shift was observed, which is typical for an absorption process involving a strong complex formation [26].
Effect of pH on the sensor response
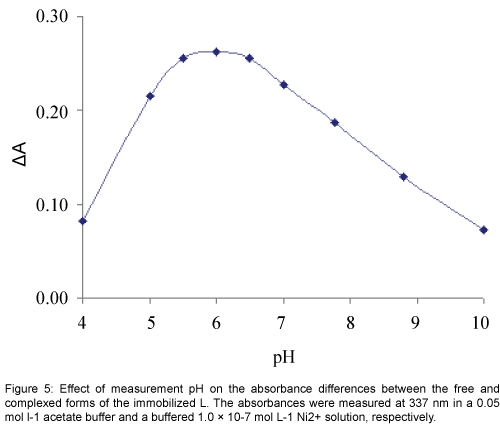
The response characteristic of the prepared membrane sensor was highly dependent to pH. Since variation of pH changed the absorbance of both the free and complexed forms of the immobilized L, for the study of the effect of pH absorbance differences (ΔA) before and after addition of Ni2+ was followed in a pH range of 4 to 10. As shown in Figure 5, the change in absorbance increased rapidly by changing the pH from 4 to about 5.5, while it was decreased at pH values higher than 6.5. The diminished response at the low pH region may be explained by the extraction of H+ from the test solution into the membrane, via protonation of the donor atoms of L, resulting in an expected change in the formation of a Ni2+-L complex. On the other hand the reduced optical response of the proposed sensor due to a possible of Ni2+ hydrolysis in higher pH values. Thus, a pH of 6.0 was considered as optimum and used for further studies [33].
Calibration curve of the sensor
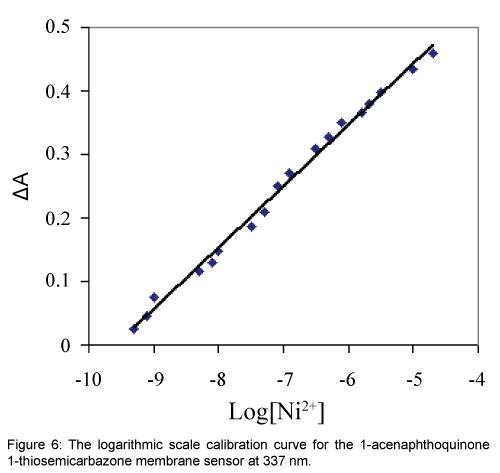
The dynamic working ranges for the proposed membrane sensor was studied by stepwise addition of Ni2+ to a series of test solutions followed by the absorbance difference monitoring at 337 nm. It was found that the absorbance decreased continuously by increasing the Ni(II) concentration and the membrane was saturated when the Ni2+ concentration exceeded 10-4 mol L-1. Under the specified experimental conditions, the calibration curve in a logarithmic scale for Ni2+ was linear from 5.01 × 10-10 to 2.04 × 10-5 mol L-1. According to the definition of IUPAC, the limit of detection (LOD, 3σ) of this method was 1.00 × 10-10 mol L-1 which is sufficiently low for Ni2+ monitoring in environmental samples [10,34,35]. The regression equation for the calibration curve shown in Figure 6 was ΔA=0.097 × Log (Ni2+)+0.925 with a correlation coefficient (R2) of 0.994.
The sensor response time
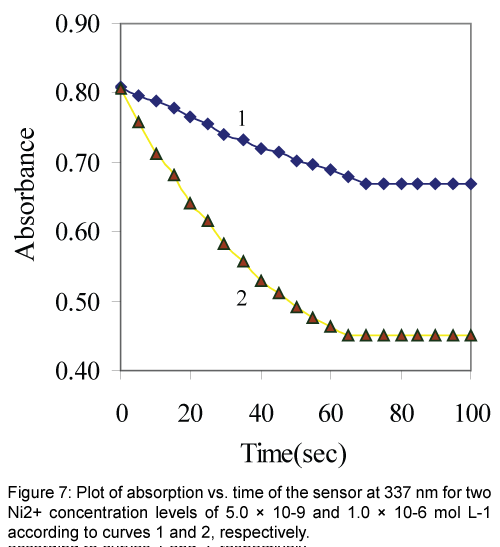
The response time of the Ni2+ sensor was calculated by plotting the absorbance as a function of time at two levels of Ni2+ concentrations. As shown in Figure 7 the profile of the response of Ni2+ optical sensor at 337 nm with time, the absorbance gets to 95% of the steady state signal in about 1 min. In general the response time decreased by increasing the analyte concentration. This may be explained by the fact that at a higher analyte concentration the rate of its diffusion in the membrane phase may be increased [10,34].
Effect of interfering ions
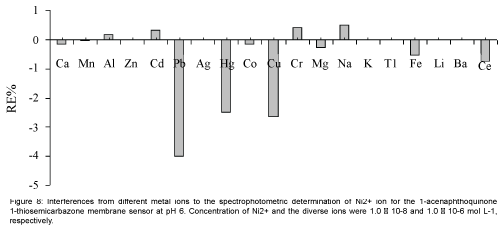
Perhaps the most important characteristics of an ion-selective optode are its selectivity, which reflects its relative response for primary ion over diverse ions present in solution. Thus, the influence of several potentially interfering ions on the response behavior of the membrane sensor was studied. To investigate the selectivity of the proposed Ni2+ optical membrane sensor, the absorbance of a fixed concentration of nickel ion, at 1.0 × 10-8 mol L-1 level, in a solution of pH=6.0 was recorded before (ΔAo) and after (ΔA) addition of some potentially interfering ions such as Ca2+, Mn2+, Al3+, Zn2+, Cd2+, Pb2+, Ag+, Hg2+, Co2+, Cu2+, Cr3+, Mg2+, Na+, K+, Tl+, Fe3+, Li+, Ba2+and Ce3+ at concentrations up to 100 times of the analyte ion. The resulting relative error is defined as RE(%)=[(ΔA-ΔAo)/ΔAo] × 100. The results of the selectivity studies are summarized in Figure 8. The data clearly indicate that, for all the studied metal ions, the relative error is up to 4%, which demonstrated that the studied interfering ions with a concentration of at least 100 times of Ni2+ ion, have no significant effect on the analytical signal [21,34,35].
Regeneration and reproducibility of the sensor
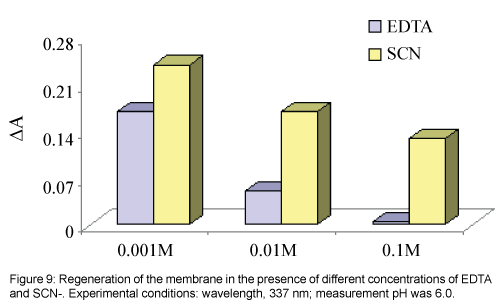
Multiple usage of an optical sensor is feasible if the sensor can be easily regenerated and give reproducible responses. Different compounds, EDTA and SCN- solutions with different concentrations were tested for regeneration of the membrane sensor and desorption of Ni2+ from it, see Figure 9. The best reagent was an EDTA solution with a concentration of 0.1 mol L-1 or higher, which can efficiently remove any adsorbed Ni2+ from the membrane and returns its absorbance to its initial value for membrane (ΔA≈0) in less than about three minute.
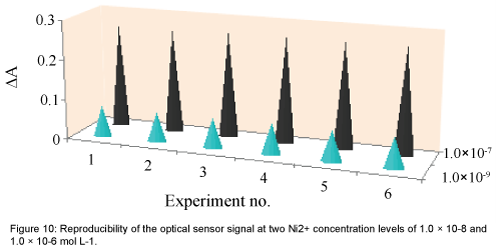
The reproducibility of the sensor response was tested by its multiple usages for Ni2+ monitoring in test solutions at two concentration levels of 1.0 × 10-9 and 1.0 × 10-7 mol L-1. After each absorbance reading, the membrane was cleaned by 0.1 mol L-1 EDTA solution, pure water and a 0.05 mol L-1 acetate buffer solution, respectively. As shown in Figure 10, good reproducibilities were obtained at both Ni2+ concentration levels. The corresponding RSD values were 1.45% and 0.83%, respectively [21,34,35].
Lifetime and stability
The life time of the membrane sensor was tested over a period of 4 months during which the membranes were stored in water [5,36-38]. The mean absorbances of the membranes at 337 nm were found to be 0.801 (± 0.025) and 0.805 (± 0.020), before and after this period, respectively. Hence, the membranes are stable within this period with a minimum life time of 4 months. Also no evidence of the ionophore leaching or signal drift was observed during multiple usages of the membrane.
Additionally, the short-term stability of the optode membrane was investigated by monitoring its absorbance values during its contact with a 1.0 10-7 M solution of Ni2+ at pH 6.0 over a period of 6 hours. From the absorbance measurements in 30 min intervals (n=2), it was found that the response was almost unchanged with only 1.0% increase in absorbance at tested wavelength after the 6 hours monitoring [34].
Application
The proposed Ni2+- selective optical sensor was used for the determination of Ni2+ in two natural water samples. The data given in Table 1 show a good agreement between the measured values using the proposed method and those obtained by the atomic absorption spectrometry (AAS) laboratory at Razi University, Kermanshah, Iran. It may be concluded that the proposed membrane sensor is selective to Ni2+ and may be used for monitoring of this ion in real water samples.
| Samples | Ni2+ (mol L-1)a | |
|---|---|---|
| Measured value (Triacetyl cellulose membrane) |
Reference value (AAS) |
|
| Gharahsoo River water | 1.68(± 0.22) × 10-7 | 1.73( ± 0.23) × 10-7 |
| Spring water | 1.21(± 0.19) × 10-7 | 1.25( ± 0.20) × 10-7 |
avalues in the parentheses are standard deviation based on the three replicate analyses
Table 1: Application of the membrane sensor for the determination of Ni2+ in different real samples.
Conclusion
The Ni2+ optical sensor that is prepared on the basis of 1-acenaphthoquinone 1-thiosemicarbazone in this study shows very good selectivity for Ni2+ over other common metal ions. The proposed sensor showed very favorable optical properties for its use as an optical sensor, such as high selectivity, adequate life time, fast and reproducible regeneration, low cost and simple fabrication and handling. The sensor can be regenerated readily with an EDTA solution and demonstrated a long life time with the possibility of multiple uses for environmental monitoring of Ni2+. Due to the advantages of the proposed method with respect to previously reported ones it may be used as an alternative method for Ni2+ determination over a range of 5.01 × 10-10 to 2.04 × 10-5 mol L-1 values without any significant interference from other metal ions.
Acknowledgements
The authors thank the Lorestan University for supporting this study.
References
- Ferreira SLC, Santos WNL dos, Lemos VA (2001) On-line preconcentration system for nickel determination in food samples by flame atomic absorption spectrometry. Anal ChimActa 445: 145-151.
- Underwood EJ (1977) Trace elements in human and Animal Nutrition. Fourth edn. Academic Press, New York, USA.
- Hu NL, Gao HW, Zhang B, Zhan GQ (2005) Simultaneous determination of cobalt and nickel in wastewater with 2-hydroxyl-5-benzeneazoformoamithiozone by spectral correction technique. J Chin ChemSoc 52: 1145-1152.
- Wu MH, Lin JL, Wang J, Cui Z, Cui Z (2009) Development of high throughput optical sensor array for on-line pH monitoring in micro-scale cell culture environment. Biomed Microdevices 11: 265-273.
- Mohr GJ, Wolfbeis OS (1994) Optical sensors for a wide range of pH based on azo dyes immobilized on a novel support. Anal ChimActa 292: 41-48.
- Wang E, Chow KF, Kwan V, Chin T, Wong C, et al. (2003) Fast and long term optical sensors for pH based on sol-gels. Anal ChimActa 495: 45-50.
- Safavi A, Bagheri M (2003) Novel optical pH sensor for high and low pH values. Sens Actuators B 90: 143-150
- Alizadeh K, Nemati H, Zohrevand S, Hashemi P, Kakanejadifard A, et al. (2013) Selective dispersive liquid-liquid microextraction and preconcentration of Ni(II) into a micro droplet followed by ETAAS determination using a yellow Schiff's base bisazanyl derivative. Mater SciEng C 33: 916-922.
- Amini MK, Khezri B, Firooz AR (2008) Development of a highly sensitive and selective optical chemical sensor for batch and flow-through determination of mercury ion. Sens Actuators B 131: 470-478.
- Hashemi P, Abolghasemi MM, Alizadeh K, AfzariZarjani R (2008) Acalmagite immobilized agarose membrane optical sensor for selective monitoring of Cu2+. Sens Actuators B 129: 332-338.
- Dridi C, Ben Ali M (2008) Electrical and optical study on modified thiacalix(4)arene sensing molecules: Application to Hg2+ ion detection. Mater SciEng C 28: 765-770.
- Yang Y, Jiang J, Shen G, Yu R (2009) An optical sensor for mercury ion based on the fluorescence quenching of tetra(p-dimethylaminophenyl)porphyrin. Anal ChimActa 636: 83-88.
- Han ZX, Luo HY, Zhang XB, Kong RM, Shen GL, et al. (2009) A ratiometricchemosensor for fluorescent determination of Hg(2+) based on a new porphyrin-quinoline dyad. SpectrochimActa A MolBiomolSpectrosc 72: 1084-1088.
- Hisamoto H, Watanabe K, Nakagawa E, Siswanta D, Shichi Y, et al. (1994) Flow-through type calcium ion selective optodes based on novel neutral ionophores and a lipophilic anionic dye. Anal ChimActa 299: 179-187.
- O’Neill S, Conway S, Twellmeyer J, Egan O, Nolan K, et al. (1999) Ion-selective optode membranes using 9-(4-diethylamino-2-octadecanoatestyryl)-acridineacidochromic dye. Anal ChimActa 398: 1-11.
- Tóth K, Thu Lan BT, Jeney J, Horváth M, Bitter I, et al. (1994) Chromogenic calix[4]arene as ionophore for potentiometric and optical sensors. Talanta 41: 1041-1049.
- Shortreed MR, Dourado S, Kopelman R (1997) Development of a fluorescent optical potassium-selective ion sensor with ratiometric response for intracellular applications. Sens Actuators B 38-39: 8-12.
- Ensafi A, Bakhshi M (2003) A stable optical film sensor based on immobilization of 2-amino-1-cyclopentene-1-dithiocarboxylic acid on acetyl cellulose membrane for Ni(II) determination. Sens Actuators B 96: 435-440.
- Shamsipur M, Poursaberi T, Karami AR, Hosseini M, Momeni A, et al. (2004) Development of a new fluorimetric bulk optode membrane based on 2,5-thiophenylbis(5-tert-butyl-1,3-benzexazole) for nickel(II) ions. AnalyticaChimicaActa 501: 55-60.
- Alizadeh N, Moemeni A, Shamsipur M (2002) Poly(vinyl chloride)-membrane ion-selective bulk optode based on 1,10-dibenzyl-1,10-diaza-18-crown-6 and 1-(2-pyridylazo)-2-naphthol for Cu2+ and Pb2+ ions. Anal ChimActa 464: 187-196.
- Shamsipur M, Hosseini M, Alizadeh K, Alizadeh N, Yari A, et al. (2005) Novel fluorimetric bulk optode membrane based on a dansylamidopropyl pendant arm derivative of 1-aza-4,10-dithia-7-oxacyclododecane ([12]aneNS2O) for selective subnanomolar detection of Hg(II) ions. Anal ChimActa 533: 17-24.
- Kim SH, Han SK, Park SH, Lee SM, Mi Lee S, et al. (1999) Use of squarylium dyes as a sensing molecule in optical sensors for the detection of metal ions. Dyes and Pigments 41: 221-226.
- Ganjali MR, Poursaberi T, Hajiagha-Babaei L, Rouhani S, Yousefi M, et al. (2001) Highly selective and sensitive copper(II) membrane coated graphite electrode based on a recently synthesized Schiff’s base. Anal ChimActa 440: 81-87.
- Gholivand MB, Ahmadi F, Rafiee E (2006) A novel Al(III)-selective electrochemical sensor based on N,N'-bis(salicylidene)-l,2-phenylenediamine complexes. Electroanalysis 18: 1620-1626.
- Ganjali MR, Rezapour M, Norouzi P, Salavati-Niasari M (2005) A new pentadentate S-N Schiffs-Base as a novel ionophore in construction of a novel Gd(III) membrane sensor. Electroanalysis 17: 2032-2036.
- Alizadeh K, Rezaei B, Khazaeli E (2014) A new triazene-1-oxide derivative, immobilized on the triacetyl cellulose membrane as an optical Ni2+ sensor. Sens Actuators B 193: 267-272.
- Hahn R, Herrmann WA, Artus GRJ, Kleine M (1995) Biologically relevant metal coordination compounds: MoVIO2 and nickel(II) complexes with tridentate aromatic Schiff bases. Polyhedron 14: 2953-2960.
- Su X, Aprahamian I (2014) Hydrazone-based switches,metallo-assemblies and sensors. ChemSoc Rev 43: 1963-1981.
- Alizadeh K, Seyyedi S, Avanes A, Ganjali MR, Faridbod F (2011) Concentration and Temperature Effects on the Electronic Absorption Spectra of 1-pyridinyl-2-methylene-benzenecarbohydrazonic Acid Following Solvatochromic Studies. ActaChimSlov 58: 251-255.
- Faridbod F, Ganjali MR, Dinarvand R, Norouzi P, Riahi S (2008) Schiff's bases and crown ethers as supramolecular sensing materials in the construction of potentiometric membrane sensors. Sensors 8: 1645-1703.
- Alizadeh K, Ghiasvand AR, Borzoei M, Zohrevand S, Rezaei B, et al. (2009) Experimental and computational study on the aqueous acidity constants of some new aminobenzoic acid compounds. J MolLiq 149: 60-65.
- Noroozifar M, KhorasaniMotlagh M, Taheri A, ZareDorabei R (2008) Diphenylthiocarbazone immobilized on the triacetyl cellulose membrane as an optical silver sensor. Turk J Chem 32: 249-257.
- Hashemi P, Hosseini M, Zargoosh K, Alizadeh K (2011) High sensitive optode for selective determination of Ni2+ based on the covalently immobilized thionine in agarose membrane. Sens Actuators B 153: 24-28.
- Alizadeh K, Parooi R, Hashemi P, Rezaei B, Ganjali MR (2011) A new Schiff's base ligand immobilized agarose membrane optical sensor for selective monitoring of mercury ion. J Hazard Mater 186: 1794-1800.
- Shamsipur M, Alizadeh K, Hosseini M, Caltagirone C, Lippolis V (2006) A selective optode membrane for silver ion based on fluorescence quenching of the dansylamidopropyl pendant arm derivative of 1-aza-4,7,10-trithiacyclododecane ([12]aneNS3). Sens Actuators B 113: 892-899.
- Ahmad M, Hamzah H, SuflizaMarsom E (1998) Development of an Hg(II) fibre-optic sensor for aqueous environmental monitoring. Talanta 47: 275-283.
- Kilian K, Pyrzyaska K (2003) Spectrophotometric study of Cd(II), Pb(II), Hg(II) and Zn(II) complexes with 5,10,15,20-tetrakis(4-carboxylphenyl)porphyrin. Talanta 60: 669-678.
- Shamsipur M, ShirmardiDezaki A, Akhond M, Sharghi H, Paziraee Z, et al. (2009) Novel PVC-membrane potentiometric sensors based on a recently synthesized sulfur-containing macrocyclicdiamide for Cd2+ ion. Application to flow-injection potentiometry. J Hazard Mater 172: 566-573.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 12259
- [From(publication date):
August-2016 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 11308
- PDF downloads : 951