Research Article Open Access
Bioremediation of Crude Oil Contamination Using Microbial Surface-Active Agents: Isolation, Production and Characterization
| Arumugam Gnanamani*, Varadharajan Kavitha, Narayanasamy Radhakrishnan and Asit Baran Mandal | |
| Microbiology Division, Central Leather Research Institute, Adyar, Chennai 20, Tamil Nadu, India | |
| Corresponding Author : | A. Gnanamani Microbiology Division Central Leather Research Institute Adyar, Chennai 20, Tamil Nadu, India Tel: 91-44-24404955 Fax: 91-44-24912150 E-mail: agmani_2000@yahoomail.com, gnanamani3@gmail.com |
| Received September 13, 2010; Accepted October 10, 2010; Published October 14, 2010 | |
| Citation: Gnanamani A, Kavitha V, Radhakrishnan N, Mandal AB (2010) Bioremediation of Crude Oil Contamination Using Microbial Surface-Active Agents: Isolation, Production and Characterization. J Bioremed Biodegrad 1:107. doi: 10.4172/2155-6199.1000107 | |
| Copyright: © 2010 Gnanamani A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The present study highlights isolation, screening, production, characterization of marine microbial surface active agents followed by evaluating the ef fi cacy of said surface -active agents for bioremediation of crude oil contamination. Upon screening, six potential surface-active agents producing isolates of Bacillus genera with signi fi cant difference in their morphology were obtained from marine sediments of Tamil Nadu coastal area. Results from optimization studies revealed, sucrose and yeast extract were the suitable carbon and nitrogen sources for growth, pH of 7.2 ± 0.2, temperature of 37ºC and agitation at 180-200 rpm were the other optimized variables for the maximum production of surface-active agents irrespective of the bacterial isolates. Extraction and characterization studies reveals, the product was polymeric in nature with the surface activity in the range of 28 ± 4 mN/m. Thermal stability was comparable with that of synthetic surfactants and exhibit appreciable emulsifying activity and emulsion stability (more than 90 days). Laboratory scale studies on removal of crude oil from aqueous phase demonstrate, >90% of crude oil was removed within 60-120 minutes of exposure to the partially puri fi ed surface-active agents. The percentage removal showed signi fi cant difference for the six different surface-active agents .
| Keywords |
| Bioremediation; Crude oil removal; Biosurfactant; Marine Bacillus sp. Surface tension; Emulsion |
| Introduction |
| Oil spills and subsequent oil removal is a major global problem. The recent oil spill tragedy in Gulf of Mexico (April 2010) spreads slowly and affects the environmentally sensitive areas too. The sudden huge volume of the oil exposure affects most of the native living populations. Though, both Government and Non- Government agencies initiated necessary steps to safe guard the lives, still, the impact has not yet retrieved completely till this date. |
| In general, surface tension of water and other liquids are reduced in the presence of synthetic organic chemicals, such as surfactants or wetting agents. Though, use of these agents may solve the above said problem, however, adding these synthetic agents to the site, in amplify the percentage level of non-degradable agents, which, further intensify the problem. These surface-active compounds, in general, contain both hydrophilic and hydrophobic (lipophilic) groups and based on their ionic behaviour in solution they are classified as anionic, cationic, non-ionic or amphoteric surfactants. The hydrophobic part of a surfactant molecule was generally derived from a hydrocarbon containing 8 to 20 carbon atoms (e.g., fatty acids, paraffin, olefins and alkyl benzenes) and the hydrophilic portion may either ionize in aqueous solution (anionic or cationic) or remain nonionic [1]. Further, recent realization on energy intensive production processes, hazardous nature [2] and the wastes generated during production restricts, use of synthetic surfactant for clinical and nonclinical applications, which necessitates the need for biocompatible and environmental benign surface-active agents. The high requirement of biosurfactants for food processing, pharmacology or solubilization of oil has been reported by number of authors. Thus, serious attention is being given at global level to have non–toxic, non-hazardous surface-active agents. |
| Microbial products of few terrestrial and most of the marine microbial species exhibit pronounced surface and emulsifying activities and are classified as bioactive compounds. Compounds that reduce surface tension at air – water interface are further grouped as biosurfactants and those involved in reducing the interfacial tension between immiscible liquids or at the solid- liquid interfaces are called as bioemulsifiers. According to Karanth et al. [3] biosurfactants usually exhibit emulsifying capacity but bioemulsifiers do not necessarily reduce surface tension. The three major important characteristics of surface-active agents; (i) enrichment at interfaces, (ii) lowering interfacial tension and (iii) micelle formation were also exhibited by the biosurfactants. |
| Most of the microbial surfactants are lipid in nature and grouped into glycolipids, phospholipids, lipopeptides, natural lipids, fatty acids and lipopolysacharides [4]. Nevertheless, the origin and the strain types decide the nature and the surface-active property of biosurfactants. According to Maneerat [5] microorganisms of marine origin exhibit the maximum yield and surface-active property compared to terrestrial species. Numbers of reports are available on glucose lipids, trehalose lipids from marine Alcanivorax borkumensis and Arthrobacter sp. ornithine lipids from marine Myroides sp. SM1, polymeric biosurfactants from marine Yarrowia lipolytica and Pseudomonas nautical and rhamnolipid from marine Pseudomonas aeruginosa A41. In addition, according to Abdul and Gibson [6] and Bai et al. [7] removal of oil/hydrocarbons from contaminated soil using commercial biosurfactants in its purified form, showing better results than that of chemical surfactants such as sodium dodecyl sulphate (SDS) and Tween 80. Similarly Urum et al. [8] reported management of crude oil in sand using commercial biosurfactants showed excellent results. However, a significant barrier to the widespread use of biosurfactant in environmental applications is the recovery of product from the production media. Santa Anna et al. [9] and Santos et al. [10] produced a crude fermented medium from Pseudomonas aeruginosa PA1 containing a blend of five types of RML, which utilized a low-cost raw material (glycerol). The use of crude biosurfactants, a viable option, limits with the research finding on the nature of biosurfactants and the organisms used for the production. |
| In the present study, we aimed to remove oil from aqueous phase using microbial surface-active agents. In detail, the study describes, isolation, production (optimization of nutrients and environmental factors) and characterization of surface-active agents from marine microbes and the efficacy of the said surface-active agents for the removal of oil from aqueous phase. |
| Materials and Methods |
| Collection and isolation |
| Marine samples of water, sediments, mussels, shells and sand were collected from Kalpakkam, Ennore port, Besant Nagar Beach, Marina beach, Mahabalipuram beach, Mandapam, Vedaranyam, Tuticorin, Cuddalore in Tamil Nadu, India, according to the procedures summarized by Saravanan et al. [11]. |
| Sabouraud’s dextrose agar (for fungi) and Zobell marine broth and agar (for bacteria) were the media used for the isolation of microbial species according to the standard procedure employed for the cultivation and maintenance of marine organisms. Fungi species were identified through observation of hyphal growth on agar plates and microscopic observation of cell dimensions. Morphologically distinct microbial colonies, screened and classified based on their Gram’s reaction. All the pure cultures are stored at - 80°C in the presence of 30% of glycerol. |
|
Screening of biosurfactants producing microorganism |
| For screening of biosurfactants producing organisms, all the obtained pure cultures were grown in Zobell medium individually at 37°C under 150 rpm for 48 h and the biosurfactants activity of the cell free medium was assessed according to the methods of Tugrul and Cansunar [12], and the isolates exhibiting appreciable surfactant activity was selected and examined for identification and further production. |
| Identification of selected isolates by phenotypical and molecular level assessment |
| Followed by screening, the selected isolates (six numbers) were subjected to phenotypical, biochemical and molecular assessment for identification. Gram staining followed by microscopical examination and all the required biochemical tests, including spore staining were carried out in addition to the 16s rDNA sequence analysis using universal primers (518F and 800R). |
| Optimization of nutritional requirements |
| Carbon: In order to assess the required carbon source and its concentration level for maximum production, to the pre-sterilized mineral medium (Sodium chloride - 19.45g; Magnesium chloride - 8.8g; Sodium sulphate - 3.24g; Calcium chloride - 1.80g; Potassium chloride - 0.55g; Sodium bicarbonate - 0.16g; Potassium bromide - 0.08g; Strontium chloride - 0.034g; Boric acid - 0.022; Sodium silicate - 0.004; Sodium fluorate - 0.0024g; Ammonium nitrate - 0.0016g; Disodium phosphate - 0.008g) supplemented, filter sterilized glucose, fructose, sucrose, glycerol at 0.5, 1.0, 1.5 and 2.0% concentrations individually and inoculated the selected isolates at 1x106 cell density and incubated at 37°C, at 180 rpm for 48 h. Followed by growth, we assessed the biosurfactants activity of the cell free medium according to the procedure summarized. |
| Nitrogen: Nitrogen in the form of beef extract, yeast extract, peptone at 0.5, 1.0, 1.5 and 2.0% concentrations were supplemented along with the mineral medium and sterilized. To the sterile medium, inoculation of the isolates and measurement of growth and biosurfactants activity made as per the procedure summarized. |
| Role of hydrocarbons: Selected isolates were grown in the mineral medium containing 2% of oils of olive, crude, sesame, peanut, sunflower, soybean and kerosene. All the required concentrations of hydrocarbons were provided to the pre-sterilized medium before inoculation. Growth and biosurfactants activity were measured as summarized. |
| Influence of environmental parameters |
| Effect of volume of the production medium: Since, volume of the medium with reference to the volume of the culture flasks play a major role in the production of biosurfactants, in the present study all the selected isolates were grown in 1000 ml of Erlenmeyer flask containing different volumes 50, 100, 150, 200, 250 and 300 ml of optimized medium. Followed by inoculation and incubation at 37°C for 48 h, the cell free medium was subjected to the measurement of surface activity according to the procedure summarized. |
| Effect of agitation: Followed by the optimization of volume, experiments were repeated again with the additional changes in the agitation at 0, 50, 100, 180 250 and 300 rpm individually and biosurfactant activity was measured as per the procedures summarized. |
| Effect of pH: In order to optimize the effective pH, isolates were grown in media containing optimized concentration of carbon and nitrogen at varied pH’s viz., 5.5, 6.5, 7.5 and 8.5. Growth and biosurfactants activity was measured at respective pH’s. In order to optimize the effective pH, isolates were grown in media containing optimized concentration of carbon and nitrogen at varied pH’s viz., 5.5, 6.5, 7.5 and 8.5. Growth and biosurfactants activity was measured at respective pH’s. |
| Effect of temperature: To have optimum temperature for maximum production of biosurfactants, cultures were grown at four different temperatures, viz., 25, 30, 37 and 40°C and measured the growth and biosurfactants activity. |
| Effect of incubation period:In order to assess the required incubation period, selected isolates were grown in the optimized media for the period of 24, 48, 72 h and the growth and biosurfactants activity was measured accordingly. |
| Production of biosurfactants |
| All the selected isolates (in the present study we used six isolates) were cultured individually in 1000 ml capacity conical flask containing optimum media volume, carbon, nitrogen, pH, temperature, agitation and incubation period. Followed by incubation, the cell free supernatant was mixed with equal amount of ice-cold ethanol and incubated for overnight. The residual pellet obtained upon centrifugation, dissolved in minimum water and considered as a partially purified sample and further subjected characterization. |
| Characterization of partially purified biosurfactants |
| Surfactant activity: Qualitative assessment of surfactant activity of biosurfactants obtained from the above experiment was made according to the methods of Tugrul and Cansunar [12] and Thaniyavaran et al. [13]. In brief, these methods describe assessment of collapse of oil drop and the relative time taken to collapse or oil displacement circle by the surface –active samples. |
| Biosurfactant activity of the samples was measured by plate method using GBX-3S tensiometer (DM) at room temperature at different dilutions ranging from 1-10 folds. In brief, 10 ml of the sample taken in a clean glass beaker (20 ml) and placed on tensiometer platform and submerged a sterile plate into solution and then slowly pulled through the liquid-air interface. Each result shown in the present study was the average of 10 determinations after stabilization. Critical Micelle Concentration (CMC) of the obtained surface-active agents was calculated accordingly. |
| Biochemical characterization of biosurfactants (BS): Biosurfactants samples were initially subjected to thin layer chromatography using various solvent combinations and spray reagents to identify their chemical nature. In brief, for amino acids, we used butanol, acetic acid, and water (4:1:1 v/v) as solvent system with ninhydrin as spraying reagent, whereas, for carbohydrates, the solvent system identified as acetonitrile: water (9: 1 (v/v)) with alpha napthol sulphuric acid as spraying reagent. Followed by TLC examination, quantitative measurements of the components of biosurfactants were made; carbohydrate concentration was quantified using phenol sulphuric method [14]; phosphate content and protein content was determined by the methods described by Hundrieser and Clark [15] and Bradford [16] respectively. |
| Hemolytic activity on human erythrocytes: To assess the hemolytic activity of biosurfactants obtained from the above experiments, we use human RBC as substrates [17,18]. RBC suspension was incubated in the presence of isotonic solution of biosurfactants solution at room temperature for 10 minutes in the dark. The amount of hemoglobin released upon centrifugation (3000 rpm) was measured at 540 nm. RBC lysis with water was taken as 100%, and sodium dodecyl sulfate (SDS) was used as reference compound [19]. |
| Antimicrobial activity: Antimicrobial activity of the biosurfactants made according to the methods of NCCLS [20] using Gram positive and Gram negative species obtained from Microbial Type Culture Collection (MTCC), Chandigarh. |
| Instrumental analysis: a) UV-Visible spectral analysis: UV–Visible spectral analysis of biosurfactants samples was made using Shimadzu UV-2450 UV– visible spectrophotometer with the spectral range of 200–800 nm. |
| b) FT-IR spectral analysis: Biosurfactants samples mixed with KBr (Sigma, US) and the pellet obtained after hydrolytic press was analyzed using Spectrum one (Perkin-Elmer Co., USA model). All measurement consisted of 500 scans and plain KBr pellet was used as the background reference. |
| c) Thermal analysis (Thermogravimetric analysis -TGA & Differential Scanning Calorimetry - DSC): Required quantity of biosurfactants sample (10-20µg) was loaded in platinum TGA pan and gravimetric analysis made under pure nitrogen atmosphere, from 0°C to 800°C using a temperature gradient of 10°C/min. Scans are routinely recorded as duplicates using TGA Q50 (V20.6 Build 31). For DSC analysis, required quantity of biosurfactant samples (10- 20µg) was loaded in aluminum DSC pan and gravimetric analysis made under reduced nitrogen atmosphere, from 0°C to 300°C using a temperature gradient of 5°C/ min. Scans are routinely recorded as duplicates using DSC Q200 (V23.10 Build 79). |
| Ionic character assessment |
| A semi quantitative agar plate method was used to determine the ionic character of the biosurfactants obtained. In brief, to the 2% agar with methylene blue (cationic dye) and pyrocatachol violet (anionic dye) incorporated individually, added 200 mg of biosurfactants to the well (0.5mm) made previously and incubated for 16- 24 h at room temperature and observed for the ring formed around the well. |
| Assessment of emulsifying activity, emulsification index and stability of emulsion of biosurfactants |
| Followed by the measurement of biosurfactant activity, we measured the bioemulsification activity of the obtained biosurfactants using heptadecane (hydrocarbon) as substrate. In brief, 50µl of the sample was mixed with 1.0 ml of Sodium acetate buffer (pH 3.0) and to this 2µl of heptadecane was added and vortexed. After 2- 3 minutes, absorbance was measured at 540 nm using UV-visible spectrophotometer (Shimadzu, Japan). One unit of emulsifying activity was defined as the amount of biosurfactant that affected an emulsion with an absorbance at 540 nm of 1.0. To assess the emulsification index, to 1.0 ml of cell free broth taken in the test tube added 1.0 ml of various hydrocarbons (oils of sesame, peanut, sunflower, soybean, rice bran and crude), benzene, toluene, and kerosene individually, vortex for 30 min and kept at room temperature. Emulsification index (E24) was calculated after 24 h, by measuring the height of emulsion layer with respect to original volume. |
| For the assessment on stability of emulsion, emulsions obtained from above experiments were kept at room temperature for the period of 0-100 days. Visual observations were made for any phase separation such as, flocculation, creamy and coalescence. Samples exhibiting nil phase separation were considered as stable emulsion. |
| Crude oil removal |
| To 100 ml of seawater taken in a 1000 ml capacity conical flask, added 10 grams crude oil followed by the addition of 10% biosurfactants and incubated under shaking (180 rpm) at room temperature for the period of 1 h. Individual experiments were carried out for all the six different biosurfactants. Rate of removal of crude oil was assessed by withdrawing the samples at every 10 minutes interval and extracted with n-hexane according to the procedure [21] and read at 400 nm. |
| Results |
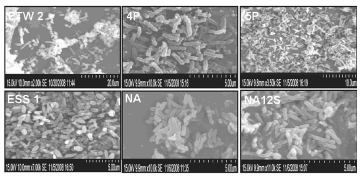
| From all the samples collected from Tamil Nadu Coastal area, we obtained two hundred morphologically distinct microbial colonies, including 194 bacteria and 6 yeasts. Out of 200 isolates, we received 18 bacterial isolates exhibiting biosurfactant activity. Further screening yields only six isolates (ETW 2, ETW 4P, ETW 5P, ESS1, ESW NA and ESW Na 12s) (ETW - samples collected from Ennore coastal area; ESS – samples from Mamallapuram coastal area; ESWsamples from Mandapam coastal area) with appreciable biosurfactant and bioemulsifying activity. Morphological examinations of six isolates reveal, isolates of ETW 4P and 5P are rough, dry, pigmented, irregular (filamentous) margin with slightly elevated convex colonies and ESW NA and NA12s are creamy, slimy and smooth colonies and ESS1 and ETW 2 are creamy, dry with regular margin. All six isolates are Gram positive with long rods of length >150 µm and breadth of > 400 nm as observed under scanning electron microscope (Figure 1). The spore staining procedures reveal, all the six isolates are spore producers. Though we have observed varied morphological features and size of the cells (SEM analysis) for all the six isolates, the Gram staining, biochemical analyses and the 16s rDNA sequence studies reveal, all the six isolates belongs to Bacillus genera. |
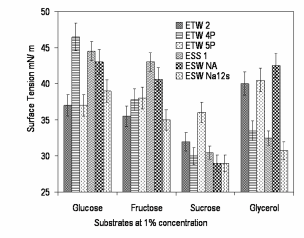
| With regard to the growth of the isolates (ETW 2, ETW 4P, ETW 5P, ESS1, ESW NA and ESW Na 12s) in the presence of different carbon sources at varied concentrations, isolates ETW 2 and ETW Na 12s grew well in the presence of sucrose at 1% concentration. With reference to biosurfactant activity, all six isolates grown in the presence of sucrose at 1% concentration reduces the surface tension of water appreciably in the average of 36 – 26 mN/m, however, isolates ESW Na and ESW Na12s still reduces the surface tension of water (72 mN/m) to 26 mN/m (Figure 2). |
| With reference to nitrogen sources, though growth of the isolates showed no significant difference between yeast and beef extract, however, surfactant property showed significant variations and we observed maximum production with high biosurfactant activity in the medium supplemented with yeast. Supplementation of peptone, though not influence growth at appreciable level but influences the surfactant activity of the isolate ESS1 and ETW 4P. |
| Further, incorporation of different hydrocarbons in the form of vegetable oils, crude oil and kerosene, cultures showed an appreciable emulsion after 48 h except, the flasks receiving kerosene. |
| Results on influence of environmental factors on biosurfactant production and activity, optimized volume of the medium for 1000ml capacity Erlenmeyer flasks was identified as 100ml and with regard to the agitation, 180 rpm was found optimum. With reference to pH, except ETW 5P, all the other five isolates showed appreciable surfactant activity (28-30 mN/m) at pH 7.2 ± 0.2. Similarly, the optimum temperature was identified as 37°C for all the isolates and a one degree increase or decrease markedly affects the biosurfactant activity. |
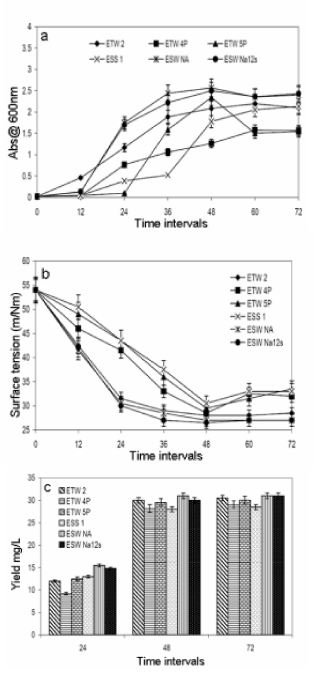
| Figure 3a illustrates growth profile of the isolates in the optimized medium conditions. Growth phase was observed till 48 h irrespective of the isolates. Furthermore, for all the isolates, pH of the medium increased slowly with respect to growth period and reached the final pH of 8.2 – 8.5. With reference to biosurfactant activity of cell free supernatant of all the six isolates grown under optimized condition, we observed all the six samples reduces the surface tension of water to lower than 30 ± 5 mN/m and the isolate ESW Na12s reduces the surface tension still lower to 25 ± 3 mN/m (Figure 3b). Figure 3c demonstrates the yield of biosurfactant with reference to incubation period for all the six isolates. Maximum yield of 30 ± 4 g/L obtained after 48 h of incubation and no significant difference in yield obtained after 48 and 72 h of incubation. |
| Followed by the production and purification, analysis of partially purified biosurfactants of six chosen isolates carried out and thin layer chromatography analysis confirms the presence of carbohydrate, protein and phosphate. The concentration of carbohydrate, protein and phosphate in mg/L was estimated as 1.8 - 8.6, 2.2- 20.2 and 2.8-38.8 respectively (Table 1a). CMC of the biosurfactants of six microbial isolates is calculated in the range of 2.301- 2.405 log of concentration. |
| With reference to hemolytic activity, no hemolysis was exhibited by the biosurfactants of six isolates. Experiments on antimicrobial activity reveal partially purified biosurfactant display no antimicrobial effect even at 100 mg/ml concentrations irrespective to target organisms. |
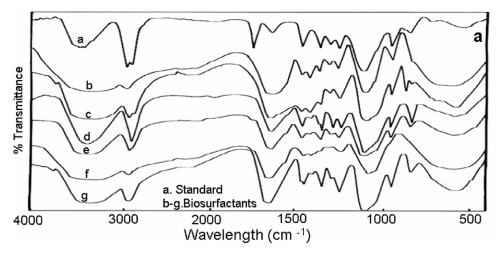
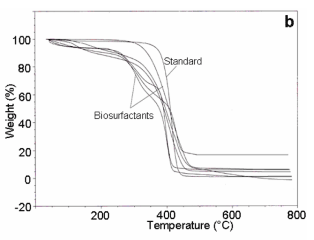
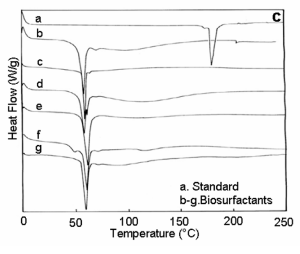
| UV-Visible spectra of the partially purified biosurfactant samples showed absorption maxima at 206 – 210nm. With respect to FT- IR analyses, presence of -OH, CH2, C=O; C=C, -CH2- COOH-, -C-H-, -P= O-, C-O- and = CH2 linkages corresponding to the peaks at 3405- 3350, 2925-2910, 1745-1725, 1660-1640, 1460-1450, 1300-1230, 1220-1210, 1100-1050, 1020-953 and 850-840 cm-1 respectively (Figure 4a). With regard to TGA analysis, all the biosurfactant samples showed stability till 220 - 280°C (Figure 4b) and DSC analysis showed a melting temperature in the range between 50-60°C (Figure 4c). With respect to ionic character of the biosurfactants, we observed no blue and brown colour ring formation around the well receiving the biosurfactants. |
| Examination of emulsification activity, emulsification index and stability of emulsion reveals, emulsion formation and stability of emulsion was high irrespective of the biosurfactant of the bacterial species. Table 1b illustrates the emulsification index of six potent biosurfactant of carbohydrate - protein nature. The emulsification index of 85-95% was observed with vegetable oils and crude oil and < 75% with kerosene. With regard to the stability of emulsions, we observed, stable emulsion formation was with vegetable oils (more than 90 days) than emulsion formed with kerosene (only up to 30 days) and crude oil. Followed by the emulsion formation, we observed a complete transformation of emulsion to thread like structures in crude oil. |
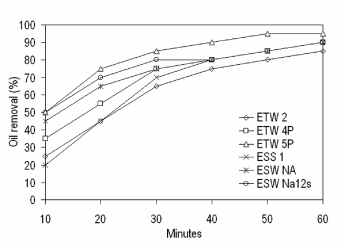
| With regard to the removal of crude oil from aqueous phase using biosurfactants of six different isolates, Figure 5 depicts percentage of crude oil removed with respect to time. More than 70% of crude oil was removed within 60 minutes of exposure to biosurfactants and >90% removal was observed after 60 minutes. |
| Discussion |
| Biosurfactants are produced by a wide variety of microorganisms, such as bacteria, yeast and fungi and the majority share goes with bacteria [22]. In the present study, we observed biosurfactants production expressed by the marine isolates of coastal Tamil Nadu was bacterial species. The chosen six isolates are Gram-positive, rod shaped and spore producing bacterial species. Their growth in the presence of 2% sodium chloride reveals they are moderate halophiles. According to Radwan and Sorkhoh [23], biosurfactants production is associated with uptake of hydrophobic carbon substrates by microorganisms. Further more, most of the reported studies emphasizes, addition of hydrophobic substrates enhance/accelerates the production of biosurfactants. Nevertheless, some organisms produce biosurfactants in the presence of water-soluble substrates, such as glucose, sucrose, glycerol, peptone [24], ethanol or other carbohydrates [25]. Wei and Chu [26] used inorganic salt-enriched medium accompanied with water-soluble substrates to achieve high biosurfactant production. In the present study, growth of the marine isolates and the biosurfactants production realized in the medium containing water soluble substrates and imply no induction or stress was required for the production. Since all the six isolates used in the present study are of marine origin, their previous and continuous exposure to oil contaminants or their inherent plasmid/chromosomal gene expressions, reasons out for their production without any inducers in the laboratory scale carried out. |
| Though, the chosen organisms are not requiring any hydrophobic substrates, however, they need soluble carbohydrates in the form of sucrose for their growth compared to glucose, fructose and glycerol. Similar observations are made by Rismani et al. [27] for the production of biosurfactants from Bacillus licheniformis. With regard to nitrogen, all the six isolates exhibit appreciable activity in the presence of yeast compared to beef and peptone. Snehal et al. [28] reported the production of biosurfactants in the presence of yeast extract and meat peptone from Rhodococcus spp. MTCC 2674. Further more, in the presence of hydrocarbons, we observed complete emulsification of the applied hydrocarbons (except kerosene) with in 36 h of incubation and suggest biosurfactants produced during the growth immediately interacts with the applied hydrocarbons. |
| Apart from carbon and nitrogen, environmental factors such as volume, aeration or agitation, incubation period, pH and temperature, also play an important role in the production and activity of biosurfactants [29]. Since all the selected six isolates obtained from 15 M distance from the seashore, the change in volume of the growth medium with respect to volume of flask and the aeration /agitation provided showed significant effect on yield and surfactant activity of the product. Volume of 100 ml of growth medium in 1000ml capacity flask considered as ideal volume and 180 rpm as optimum agitation for the maximum production and surfactant activity. However, observations on reduction in surfactant activity with an increase in agitation above 200 rpm, reasoned to the reduced uptake of nutrients during agitation condition. Syldatk et al. [29] also found increase in agitation speed from 250 to 500 rpm decreases the production of biosurfactant in R.erythropolis. |
| With regard to the pH of the growth medium on production of surface-active agents from bacterial species, a wide variation in production is reported by number of authors [30,31]. Rhamnolipid production by Pseudomonas sps is high at pH range from 6 to 6.5 and decreased sharply above pH 7.0 [31]. In the present study, production and activity of the biosurfactant found maximum at pH 7.0, and an additional increase in pH during bacterial growth, does not affect the surfactant activity till pH 8.5 ± 0.2, however, decrease in pH showed significant effect on the surfactant activity. This could be reasoned to the nature and the source of origin of the microbial species employed. The optimum temperature observed as 37°C irrespective of the isolates and further increase in temperature results with a decrease in growth as well surfactant activity, suggests, temperature play a significant role in the production of biosurfactants. Syldatk et al. [29] found Pseudomonas sp. showed maximum growth and surfactant activity when grown at 37°C. |
| Though numbers of methods (acid precipitation, solvent extraction, crystallization, ammonium sulfate precipitation) are reported for the recovery of biosurfactants, in the present study we attempted both acid precipitation and solvent extraction during the initial period of experiments and based on the yield and activity; we followed only solvent extraction procedure for recovery of biosurfactants. Furthermore, observations on insignificant difference in the yield of the biosurfactants of six isolates, despite the difference in their phenotype, suggests, isolates of same origin might have similar expressions. Yield of about 4.0-5.0 g/ L (on dry weight basis) was received for each isolates. Pseudomonas aeruginosa strain BS2 yields 0.92 g/L of biosurfactants, when curd whey as substrate. Bacillus subtilis ATCC 21332 yields 2.2-3.0 g/L of biosurfactant, when cassava flour wastewater was used as substrate. With reference to the activity of the biosurfactants, according to Mulligan [32] a good surfactant can lower the surface tension of water from 72 to 35 mN/m. In the present study, surfactant activity of all the six biosurfactants reduces the surface tension of water from 72 to 28 ± 4 mN/m. Thus, the obtained surfactant can be grouped under good/ very good biosurfactants. |
| Results on characterization of biosurfactants reveal, obtained biosurfactants of all the six bacterial isolates contains carbohydrates, proteins and phosphate at considerable concentrations. Further UV- visible and FT-IR spectral analyses showed presence of protein compounds (a small hump in the region of 280 - 290 nm) and amide I linkages with C=O stretching (1653 cm-1) and amide- II linkage due to coupling of N-H bending and C-N stretching followed by a aliphatic P=O stretching (1260-1240 cm-1), evidently proves the said constituents. Based on this information, we proposed a hypothetical structural elucidation which implies, the obtained biosurfactants consists of a maximum percentage of oligopeptides containing - leuisoleu- ala-met-: a series of amino acids followed by repeating units of deoxymannose with phosphate moiety. Moreover, due to this polymeric nature, these biosurfactants exhibit high stability with low melting point as shown in Figure 4b and 4c. Nitschke and Pastore [33] reported biosurfactant of B. subtilis LB5a showed stability after 121°C. However, more elucidations are still required to confirm the structure of the biosurfactants extracted from marine bacterial isolates. Husain et al. [34] reported the production of polymeric biosurfactant by Pseudomonas nautical. Experiments on ionic characterization of biosurfactants of six isolates reveals, all the six biosurfactants are grouped under non-ionic. Lang and Philp [35] reported nonionic biosurfactants of Rhodococcus erythropolis bacterial species. Further, this non-ionic behaviour could be one of the reasons for their non-hemolytic activity against RBC’s tested in the study. Nakar and Gutnick [36] reported some biosurfactants; especially those with nonionic properties are not likely to cause hemolytic. Although results on antimicrobial activity of various biosurfactants are reported in the literatures [37] none of our biosurfactants exhibit antimicrobial property tested against standard bacterial strains. This also could be due to the non-ionic behaviour of the biosurfactants. |
| Further, studies on emulsification index and emulsion stability reveals, irrespective of the biosurfactants obtained from six different species, stability in emulsion with chosen hydrocarbons was observed for more than 90 days. No appreciable results on emulsion formation with kerosene. In contrary, Haba et al. [38] reported a stable emulsion with kerosene in the presence of bioemulsifier obtained from Pseudomonas aeruginosa 47T2 for the period of 90 days. |
| Experiments on crude oil removal study demonstrate marine microbial-based surface-active agents able to remove the crude oil within a very short period of 60 minutes. Higher removal percentage observed in the presence of biosurfactant attributed to the interaction of surfactant-water and surfactant-oil (such as interfacial tension reduction) [38], which dominates the interaction of oil-water and further attributed to the reduction of surface and interfacial tensions of surfactant solutions and increases the mobility of oil and consequently enhances the separation of oil from aqueous solution. |
| Biosurfactants of marine microbial origin demonstrates removal of crude oil from aqueous phase within a short period of time and further emulsify the vegetable oils. This property of the obtained biosurfactants was highly encouraging at the present day concept of oil spills. |
| Followed by our continuous observations on reproducibility and the potency, the most important isolates were deposited in IMTECH (Institute of Microbial Technology, Chandigarh) with accession numbers, MTCC 5514, MTCC 5513 for public domain accessibility. The sequence details are submitted in NCBI GenBank collection with accession numbers as HM222944, HM145910, and HM194725. In addition, a national patent was filed for the process and the product obtained as DEL 1261/2010. |
| Acknowledgements |
| All the authors acknowledge the Department of Biotechnology, Ministry of Science and Technology, New Delhi, India for their financial support in the form of project (BT/PR8204/AAQ/03/302/ 2006). |
References
|
Tables and Figures at a glance
| Table 1a | Table 1b |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4a |
 |
 |
 |
| Figure 4b | Figure 4c | Figure 5 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 16784
- [From(publication date):
November-2010 - Dec 06, 2025] - Breakdown by view type
- HTML page views : 11888
- PDF downloads : 4896
